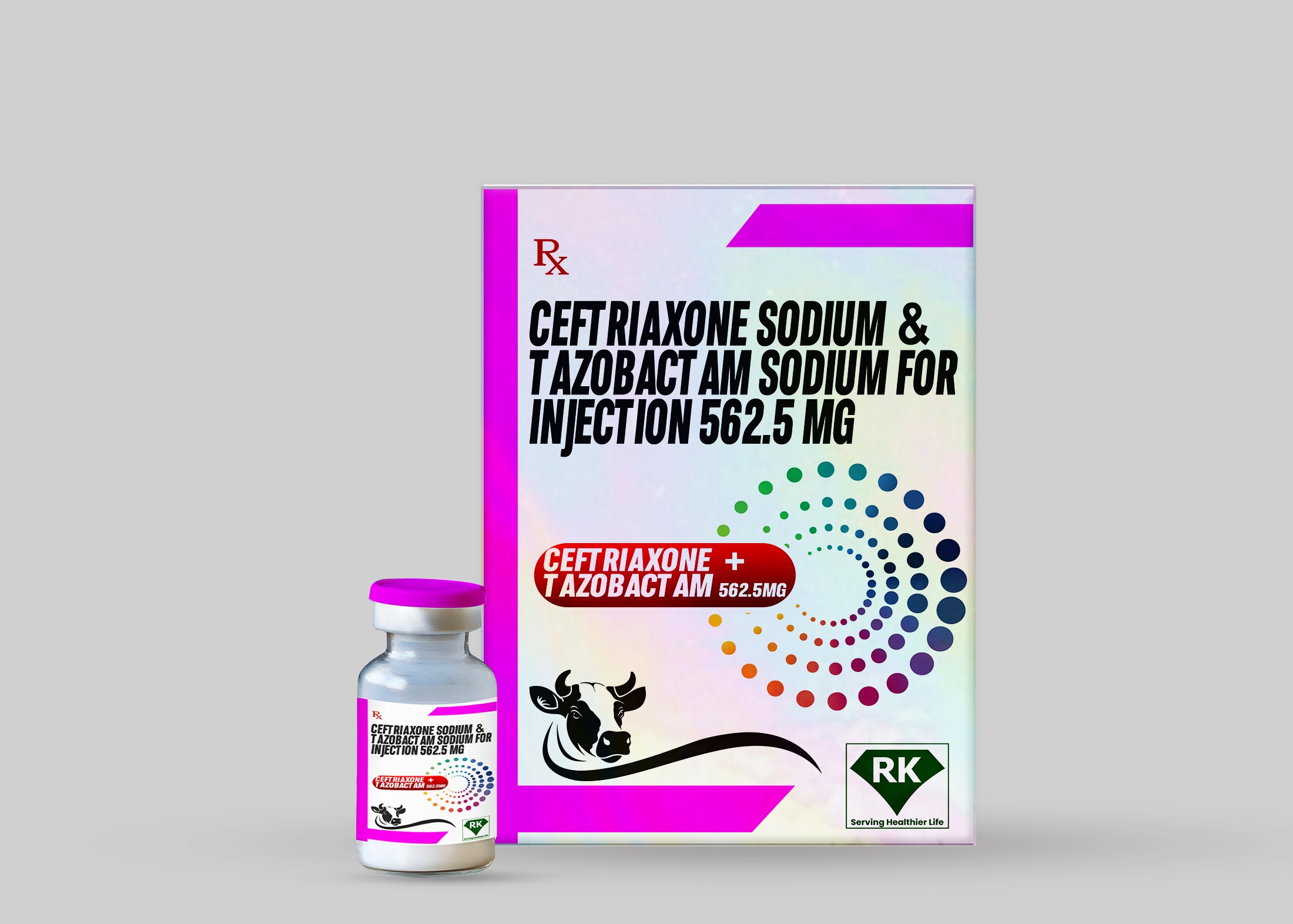
R K Lifecare Inc: Leading Manufacturer and Wholesaler of Ceftriaxone Sodium and Tazobactam Sodium for Injection 562.5mg
At R K Lifecare Inc, We take great pride in being one of the leading Ceftriaxone Sodium and Tazobactam Sodium for Injection 562.5mg manufacturers in India, providing essential antibiotic therapy to healthcare providers worldwide.
Our company specializes in the production and distribution of Ceftriaxone Sodium Injection and Tazobactam Sodium Injection, two leading antibiotics that work synergistically to treat various bacterial infections. We are committed to ensuring that high quality medicines such as Ceftriaxone Sodium Injection and Tazobactam Sodium Injection are readily available to hospitals, clinics and healthcare providers, whether located in India or abroad.
As one of the top Ceftriaxone Sodium Injection manufacturers in India, We have built a reputation for consistently delivering products that meet the highest standards of efficacy and safety. Our team at R K Lifecare Inc is dedicated to advancing healthcare by manufacturing products that provide effective treatment for serious bacterial infections, ensuring the well-being of patients.
Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg is a combination of two powerful antibiotics. Ceftriaxone, a third-generation cephalosporin, is effective against a wide range of bacterial pathogens, while tazobactam acts as a beta-lactamase inhibitor, enhancing the activity of ceftriaxone against resistant bacteria. This combination therapy is invaluable in treating infections that are resistant to other antibiotics, making it a cornerstone of modern antimicrobial treatment.
At R K Lifecare Inc, Our goal is to provide the healthcare industry with a reliable source of Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg, ensuring that healthcare providers have access to this lifesaving medication when they need it most.
As a leading Ceftriaxone Sodium Injection wholesaler in India, We are committed to meeting the demand for these injections, not only locally but also through our extensive distribution network. Our strategic position in the industry as a reliable Ceftriaxone Sodium Injection supplier in India allows us to reach out to healthcare providers across India, including the Delhi NCR region, and supply them consistently and on time.
Our company has a vast distribution network with established relationships with Ceftriaxone Sodium Injection distributors in India and Tazobactam Sodium Injection distributors in India. This helps us maintain a steady supply of Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg, and ensures that healthcare professionals even in remote areas can access these essential antibiotics.
We understand the critical nature of timely medication, and our team works round the clock to ensure that we can meet the needs of Ceftriaxone Sodium Injection dealers and healthcare providers across the country. From the procurement of raw materials to the manufacturing and distribution of the final product, we maintain the highest standards in quality control (QC) and regulatory compliance.
Ceftriaxone Sodium and Tazobactam Sodium 562.5mg for Injection is designed to treat a wide range of infections, including respiratory tract infections, urinary tract infections, intra-abdominal infections, and skin and soft tissue infections. These infections, caused by both gram-positive and gram-negative bacteria, are often complex and require a combination of antibiotics to achieve optimal results.
By combining Ceftriaxone Sodium with Tazobactam Sodium, our formulation addresses a wide range of bacterial pathogens, including those that have developed resistance to other antibiotics. As a leading manufacturer and Tazobactam Sodium Injection wholesaler in India, R K Lifecare Inc is proud to be part of the global effort to combat the growing challenge of antibiotic resistance.
With a commitment to quality and patient safety, we ensure that our injections are manufactured under the most stringent guidelines to maintain their efficacy and safety. Manufacturing Process of Ceftriaxone Sodium and Tazobactam Sodium 562.5mg for Injection
Manufacturing Ceftriaxone Sodium and Tazobactam Sodium 562.5mg for Injection involves a precise and systematic process, to ensure that the final product is of the highest quality and meets the regulatory requirements for drug manufacturing.
Procurement of Raw Materials
The first step in the manufacturing process is the procurement of raw materials. At R K Lifecare Inc, We ensure that the raw materials used in the production of Ceftriaxone Sodium and Tazobactam Sodium 562.5mg for Injection are obtained from certified suppliers who comply with the best practices in the industry.
These raw materials include the active pharmaceutical ingredients (APIs) ceftriaxone and tazobactam, as well as the excipients required for manufacturing. Quality is our top priority, and we follow stringent standards to select our suppliers and ensure the purity and quality of each ingredient used in our products.
Raw Material Quality Control (QC)
Once the raw materials are procured, they must undergo quality control (QC) testing to verify their identity, purity, and potency. This is a critical step in the manufacturing process, as it ensures that only the highest quality ingredients enter production.
Each batch of raw materials is tested for consistency and compliance with pharmacopoeial standards before being used in the manufacture of Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg. Our in-house QC team conducts rigorous testing and reviews all results before approving the ingredients for further processing.
Formulation and Blending
Once the raw materials are approved, the next step is formulation and blending. This involves precisely mixing Ceftriaxone Sodium and Tazobactam Sodium with other excipients to create the final injectable solution. The formulation process must be performed under strict sterile conditions to ensure that no contaminants are introduced.
Our team of experienced formulators ensures that the active ingredients are evenly distributed throughout the solution to guarantee the correct dosage in each vial. The mixing process is carefully controlled to maintain consistency and accuracy in each batch of Ceftriaxone Sodium and Tazobactam Sodium for Injection 562.5mg.
Sterile Filtration and Filling into Vials
Once the formulation is complete, the next step is sterile filtration. This process removes any particulate matter or microorganisms that could potentially contaminate the injection. The solution is passed through special filters to ensure it is sterile before it is filled into vials.
Filling into vials is done using advanced, automated equipment designed to minimize human error and contamination. Each vial is filled with a precise dose of Ceftriaxone Sodium and Tazobactam Sodium 562.5mg for Injection, ensuring that healthcare providers can administer the exact amount needed for effective treatment.
Lyophilization (Freeze Drying)
For some injectable products, lyophilization (freeze drying) is used to preserve the active ingredients and increase shelf life. This process involves freezing the solution and then removing the water content under vacuum. The resulting freeze-dried product is more stable and easier to store. Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg undergoes lyophilization to ensure that the drug remains potent and effective even after long periods of storage.
Stoppering and Sealing
Once the vials are filled and lyophilized (if required), they undergo a stoppering and sealing process. This is done to ensure that the drug remains sterile and free from contamination during storage and transportation. The vials are sealed with rubber stoppers and aluminum caps to protect the integrity of the product. This step is critical to maintaining the shelf life and safety of the injection.
Quality Control (QC) and Quality Assurance (QA) Testing
After the manufacturing process is complete, each batch of Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg undergoes rigorous QC and QA testing. This includes testing for potency, purity, sterility and overall product quality. Our in-house QC team performs multiple tests to ensure that each batch meets the required standards before it is released for distribution. We follow international guidelines and regulations to ensure that our products are safe, effective and in line with industry standards.
Uses and Side Effects
Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg is used to treat a wide range of bacterial infections including respiratory tract, urinary tract, intra-abdominal infections and skin and soft tissue infections. The combination of ceftriaxone, a broad-spectrum cephalosporin and tazobactam, a beta-lactamase inhibitor provides better coverage against resistant pathogens, making it an effective option for patients who may not respond to other antibiotics.
However, like all medications, Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg can cause side effects. Common side effects include gastrointestinal symptoms such as nausea, vomiting, and diarrhea, as well as reactions at the injection site. Serious side effects, although rare, may include allergic reactions, liver dysfunction, and blood disorders. It is important for healthcare providers to monitor patients for potential adverse effects during treatment.
Labeling and Packaging
The labeling and packaging of Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg is done with attention to detail to ensure that all necessary information is clearly provided. Each vial contains the correct dosage, instructions for use, expiration date, and warnings. The packaging is designed to protect the product from damage and contamination during transit.
Storage and Storage
Proper storage and handling is essential to maintain the quality and potency of Ceftriaxone Sodium and Tazobactam Sodium Injection 562.5mg. Our storage facilities are temperature-controlled to ensure that products remain effective throughout their shelf life. We also use advanced inventory management systems to keep track of stock and ensure that products are promptly available to meet demand.

22 Jan 2026
20 Jan 2026

19 Jan 2026

13 Jan 2026

08 Jan 2026
.jpg)
.jpg)
.jpg)
.jpg)