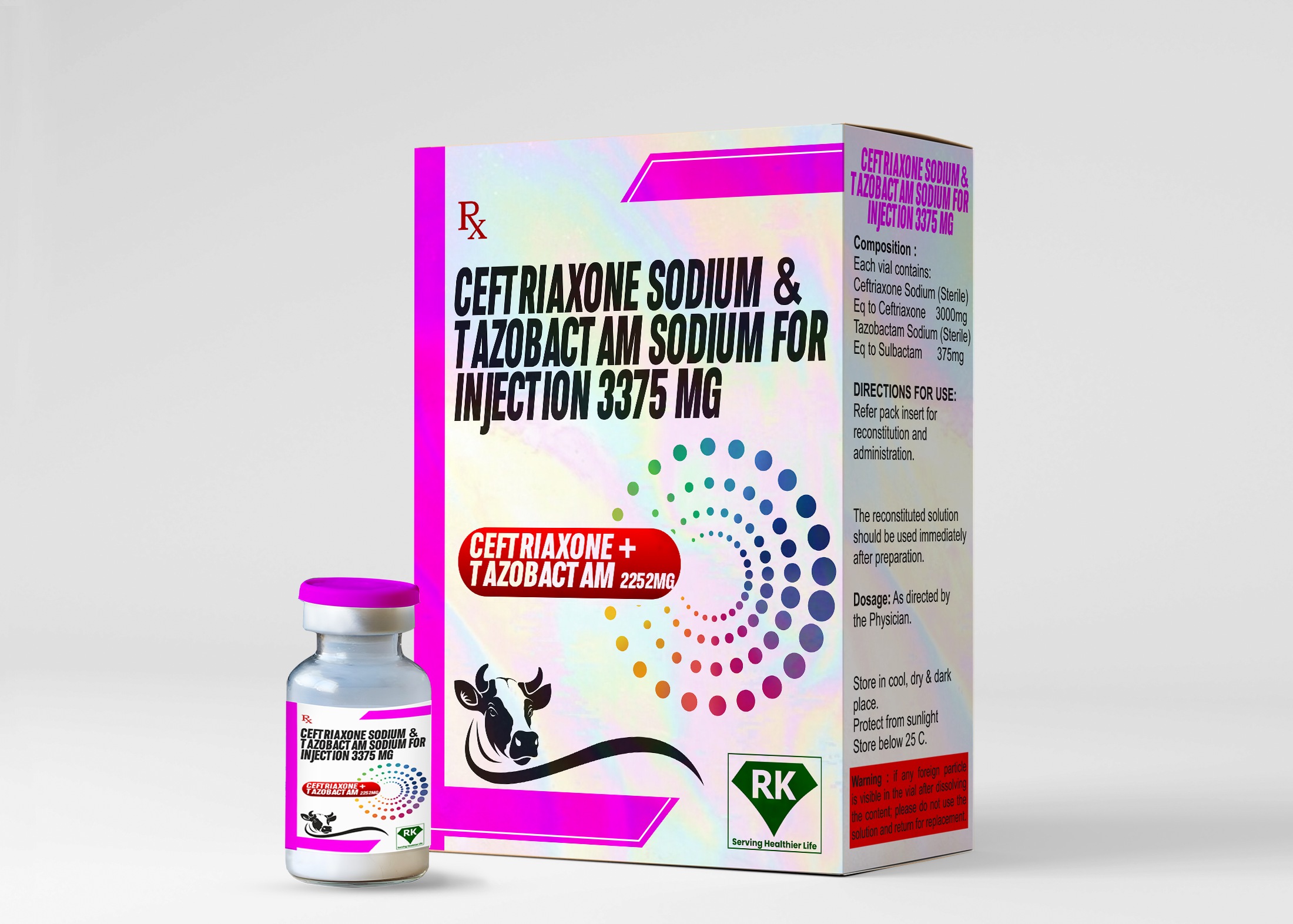
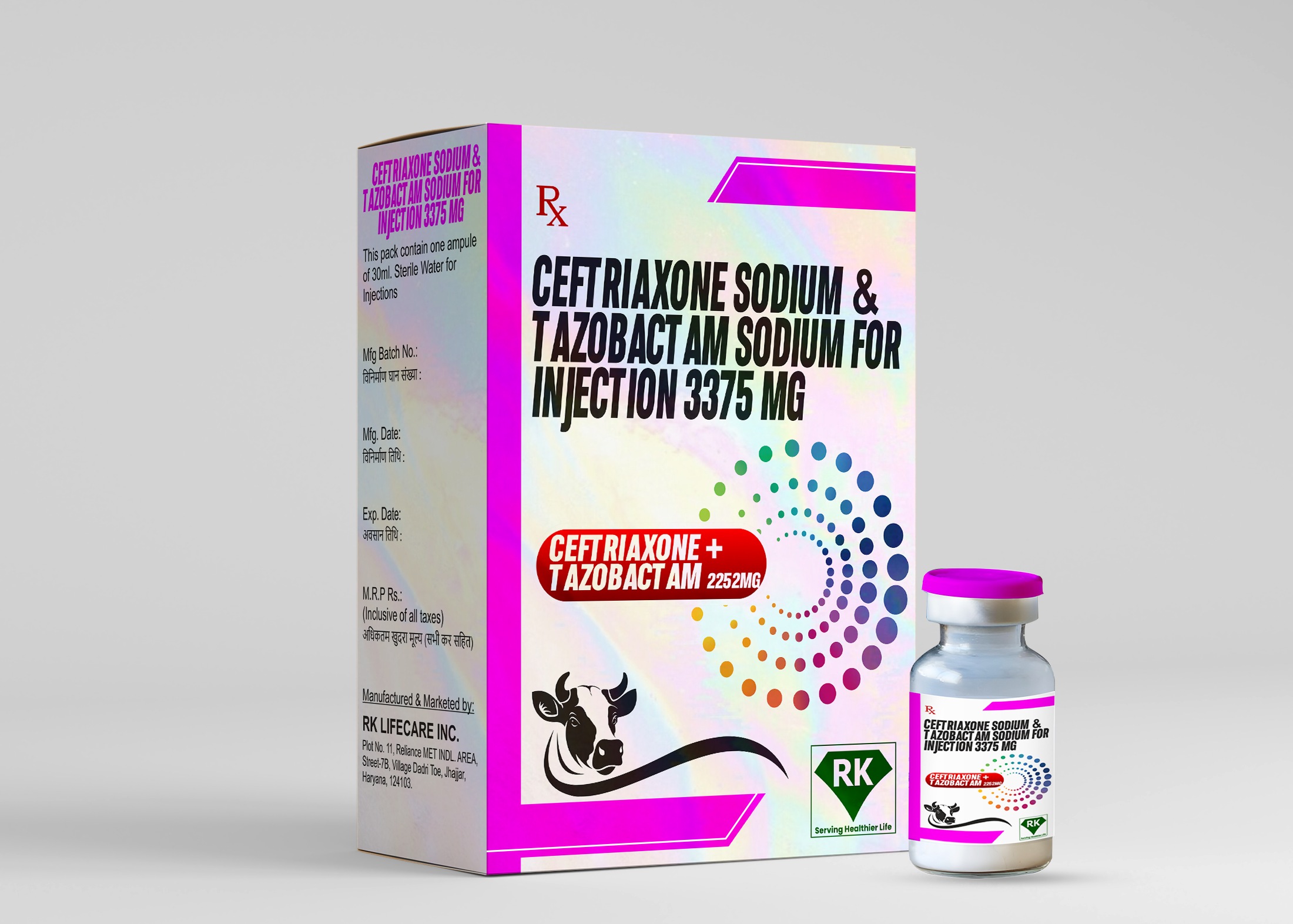
Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg: Leading Manufacturer and Wholesaler at R K Lifecare Inc.
At R K Lifecare Inc, We are committed to excellence in the manufacturing and wholesale distribution of Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg, which play a vital role in providing these essential life-saving medicines to healthcare providers in India and beyond.
As a reliable and trustworthy manufacturer, we have established ourselves as one of the leading Ceftriaxone Sodium Injection manufacturers in India and Tazobactam Sodium Injection manufacturers in India, serving a variety of medical facilities that require high-quality injectable antibiotics to combat serious bacterial infections.
Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg is a combination of two powerful drugs: ceftriaxone, a third-generation cephalosporin antibiotic, and tazobactam, a beta-lactamase inhibitor. The combination of these two components makes the injection effective against a wide range of bacterial pathogens, including pathogens that produce an enzyme called beta-lactamase, which can render many antibiotics ineffective. Our ability to manufacture these drugs in high volumes and distribute them efficiently is what sets us apart in the pharmaceutical industry.
We are proud to be one of the most reliable Ceftriaxone Sodium and Tazobactam Sodium wholesalers for injection in India, ensuring that our products are available in hospitals, clinics, and pharmacies across the country. As a Ceftriaxone Sodium Injection distributor in India and Tazobactam Sodium Injection distributor in India, we understand the importance of timely delivery, especially when it comes to critical drugs such as these.
Healthcare providers rely on a steady supply of quality antibiotics, and at R K Lifecare Inc, We work tirelessly to ensure that our products reach every corner of India, including areas such as Ceftriaxone Sodium Injection in Delhi NCR. By partnering with Ceftriaxone Sodium Injection dealers and Tazobactam Sodium Injection dealers, we expand our reach and make these lifesaving medications accessible to even more healthcare providers.
As one of the leading Ceftriaxone Sodium Injection suppliers in India and Tazobactam Sodium Injection suppliers in India, we focus on delivering these medications with a commitment to quality and integrity. With extensive experience in the pharmaceutical manufacturing process, we are dedicated to producing Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg that meets rigorous global standards. Our facilities are equipped with advanced technology and comply with the highest manufacturing practices to ensure that every batch of injections is safe, effective, and of the highest quality.
Our commitment to quality is reinforced by our strict adherence to quality control (QC) and quality assurance (QA) standards, ensuring that each vial of Ceftriaxone Sodium and Tazobactam Sodium Injection undergoes rigorous testing before it is released to the market.
The combination of Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg is often prescribed in hospitals and critical care units to treat various infections caused by sensitive bacteria. These infections may include severe respiratory infections, urinary tract infections, intra-abdominal infections, skin and soft tissue infections, etc. The combination of Ceftriaxone Sodium and Tazobactam Sodium helps address both the bacteria’s ability to resist antibiotics and the underlying infection.
Given the increasing prevalence of antibiotic-resistant bacteria, this combination therapy is an important tool in managing infections that would be difficult to treat with other antibiotics. As a Ceftriaxone Sodium and Tazobactam Sodium Injection manufacturer in India, we are proud to be at the forefront of providing this critical drug to healthcare professionals who depend on these drugs to save lives. Sterile Filtration and Filling into Vials
The production process of Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg begins with sterile filtration. In our state-of-the-art manufacturing facilities, we use advanced filtration techniques to remove any potential contaminants or microorganisms from the solution. This step is crucial in ensuring that the final product is sterile and safe for patient use. After sterile filtration, the solution is carefully filled into vials in a controlled and aseptic environment.
The filling process ensures that each vial contains the correct dosage of Ceftriaxone Sodium and Tazobactam Sodium and is ready for use in clinical settings. As a reliable Ceftriaxone Sodium Injection wholesaler in India, we understand the importance of precision and care at every stage of production to ensure the highest quality final product. Lyophilization (Freeze Drying)
In some cases, Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg is lyophilized (freeze-dried) to improve its stability and extend its shelf life. Lyophilization involves freezing the solution and removing the water content under vacuum conditions. This process preserves the active ingredients while maintaining their potency and effectiveness. The freeze-dried product is then repackaged and stored in vials, where it can be reconstituted with the proper diluent when it is time for administration.
This method is commonly used in injectable medications to ensure that the product remains stable during transportation and storage. As Tazobactam Sodium Injection manufacturers in India, we use this advanced technology to ensure that the final product maintains its therapeutic properties for a long time. Stoppering and Sealing
After filling Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg into vials and, if necessary, freeze-drying, the next step is the stoppering and sealing process. In this step, the vials are sealed with a stopper to prevent contamination from the environment.
The sealing process is performed under highly controlled conditions to ensure that no air or moisture enters the vial, which could potentially affect the stability of the drug. We use high quality stoppers and sealing equipment to ensure that the vials remain airtight and the product remains sterile until it is ready for use. This process is critical to maintaining the integrity of Ceftriaxone Sodium and Tazobactam Sodium Injection throughout its shelf life.
Quality Control (QC) and Quality Assurance (QA) Testing
At R K Lifecare INC, Quality is the core of everything we do. We are committed to producing only the highest quality Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg, and to achieve this, we implement stringent Quality Control (QC) and Quality Assurance (QA) measures. Before any batch of Ceftriaxone Sodium and Tazobactam Sodium Injection is released to the market, it undergoes a detailed set of QC and QA tests. These include tests for sterility, potency, pH levels, and microbiological contamination.
Our QA team ensures that all regulatory requirements are met, and every step of the manufacturing process follows industry best practices. Only after passing all these tests is the product approved for distribution. As Ceftriaxone Sodium Injection manufacturers in India, our commitment to quality ensures that healthcare professionals can rely on our products to deliver consistent and reliable results. Uses and Side Effects
Ceftriaxone Sodium and Tazobactam Sodium for Injection 3375mg is used to treat a wide range of bacterial infections, including respiratory infections, urinary tract infections, intra-abdominal infections, skin and soft tissue infections, and more.
This combination therapy is effective against both gram-positive and gram-negative bacteria, including bacteria that produce beta-lactamase enzymes, which can otherwise render certain antibiotics ineffective. It is commonly used in hospitals, especially in intensive care units, to manage severe and life-threatening infections.
However, like any medication, it has potential side effects. The most common side effects of Ceftriaxone Sodium and Tazobactam Sodium for Injection include local reactions at the site of injection, such as pain or swelling.
Other side effects may include allergic reactions, nausea, diarrhea, or changes in liver enzymes. While these side effects are typically mild and transient, more serious reactions may occur in rare cases, such as anaphylaxis or severe gastrointestinal disturbances. Healthcare providers should monitor patients closely during treatment and take appropriate action if any adverse effects occur.
Labeling and Packaging
Proper labeling and packaging are essential components of the drug manufacturing process. At R K Lifecare Inc, We ensure that each vial of Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg is clearly labeled with all necessary information, including dosage, storage conditions, expiration date, and batch number.
The packaging is designed to protect the vials from damage during transportation and storage. We use high-quality packaging material that meets industry standards, ensuring that the product reaches healthcare providers in optimal condition. As a leading Tazobactam Sodium Injection wholesaler in India, we understand the importance of clear labeling and secure packaging in maintaining product quality and safety.
Storage and Warehouse
Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg must be stored in a controlled environment to maintain its stability and potency. At R K Lifecare Inc, We maintain state-of-the-art storage facilities that meet strict regulatory requirements. Our warehouses are temperature controlled to ensure that the product remains within the recommended storage limits. Additionally, our inventory management system tracks each batch to ensure that the products are used within their expiry dates and are kept in optimal conditions.
Distribution and Supply Chain
As a Ceftriaxone Sodium Injection supplier in India and Tazobactam Sodium Injection supplier in India, we have developed a highly efficient distribution and supply chain process. We work closely with our network of distributors, wholesalers and dealers to ensure that Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg reaches healthcare facilities across India and beyond. Our supply chain is designed to minimize delays and ensure timely delivery of products, even in remote areas. We understand that the demand for antibiotics like Ceftriaxone Sodium and Tazobactam Sodium is often urgent, and our distribution system is optimized to meet those needs.
Procurement of Raw Materials
The production of Ceftriaxone Sodium and Tazobactam Sodium Injection 3375mg begins with the careful procurement of high-quality raw materials. We source our active pharmaceutical ingredients (APIs) and excipients from trusted suppliers that meet our stringent quality standards. Each batch of raw materials is thoroughly tested before being used in production, to ensure that the final product meets the required specifications.
Quality Control (QC) of Raw Materials
Before any raw materials are used in production, they are required to undergo extensive quality control tests to ensure their purity and potency. These tests are crucial in preventing any contaminants or substandard materials from being introduced into the manufacturing process. Our QC team works closely with our suppliers to ensure that only the highest quality raw materials are used in the production of Ceftriaxone Sodium and Tazobactam Sodium for Injection.
Formulation and Blending
Once the raw materials pass the QC testing, they are carefully blended to create the final formulation for Ceftriaxone Sodium and Tazobactam Sodium 3375mg for Injection. This step ensures that the active ingredients are evenly distributed throughout the solution, providing a uniform and effective dosage in every vial. After mixing, the product undergoes further testing to ensure that it meets the required potency and stability before moving on to the next step of the manufacturing process.
08 Dec 2025
03 Dec 2025

02 Dec 2025

28 Nov 2025

31 Oct 2025