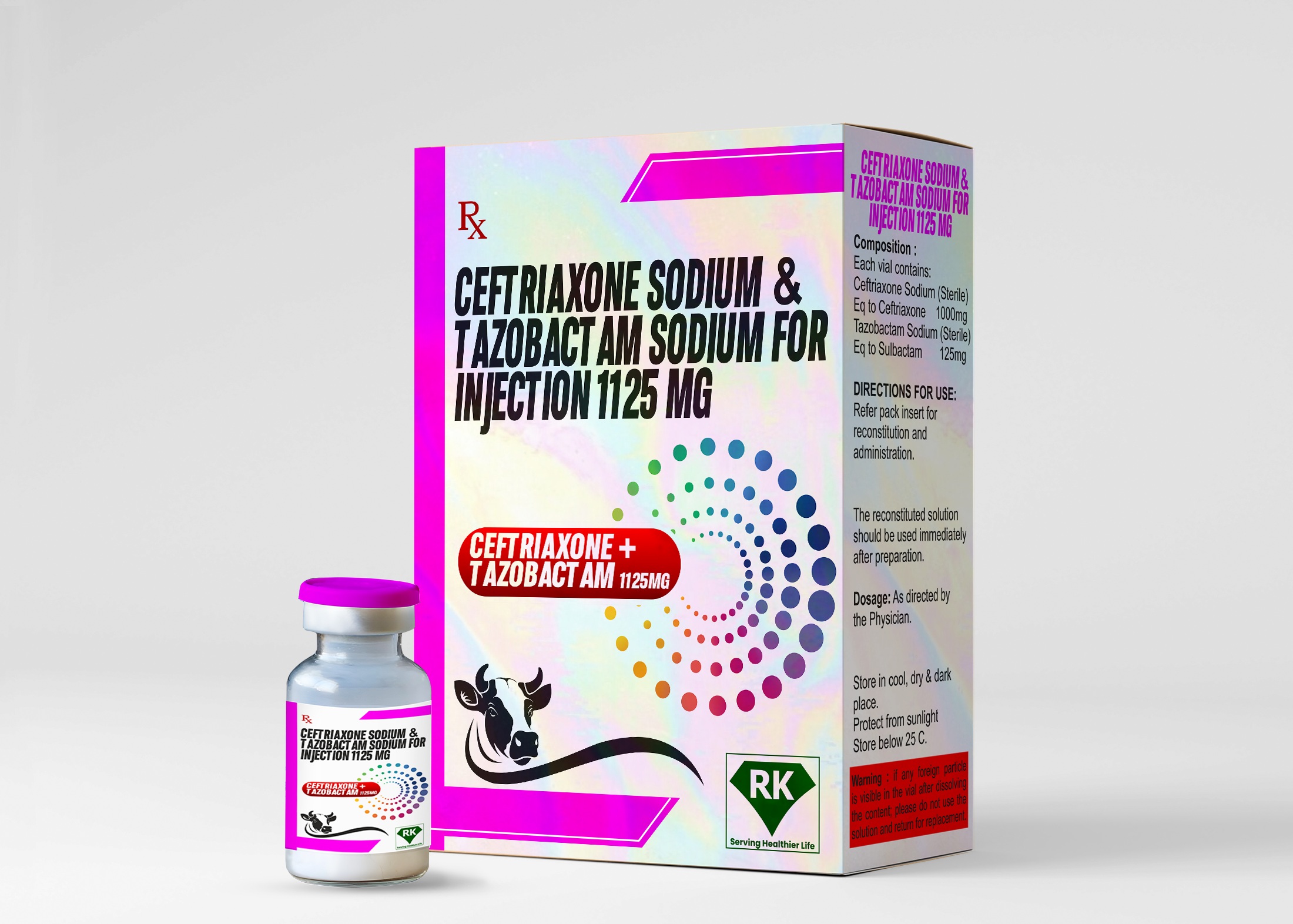
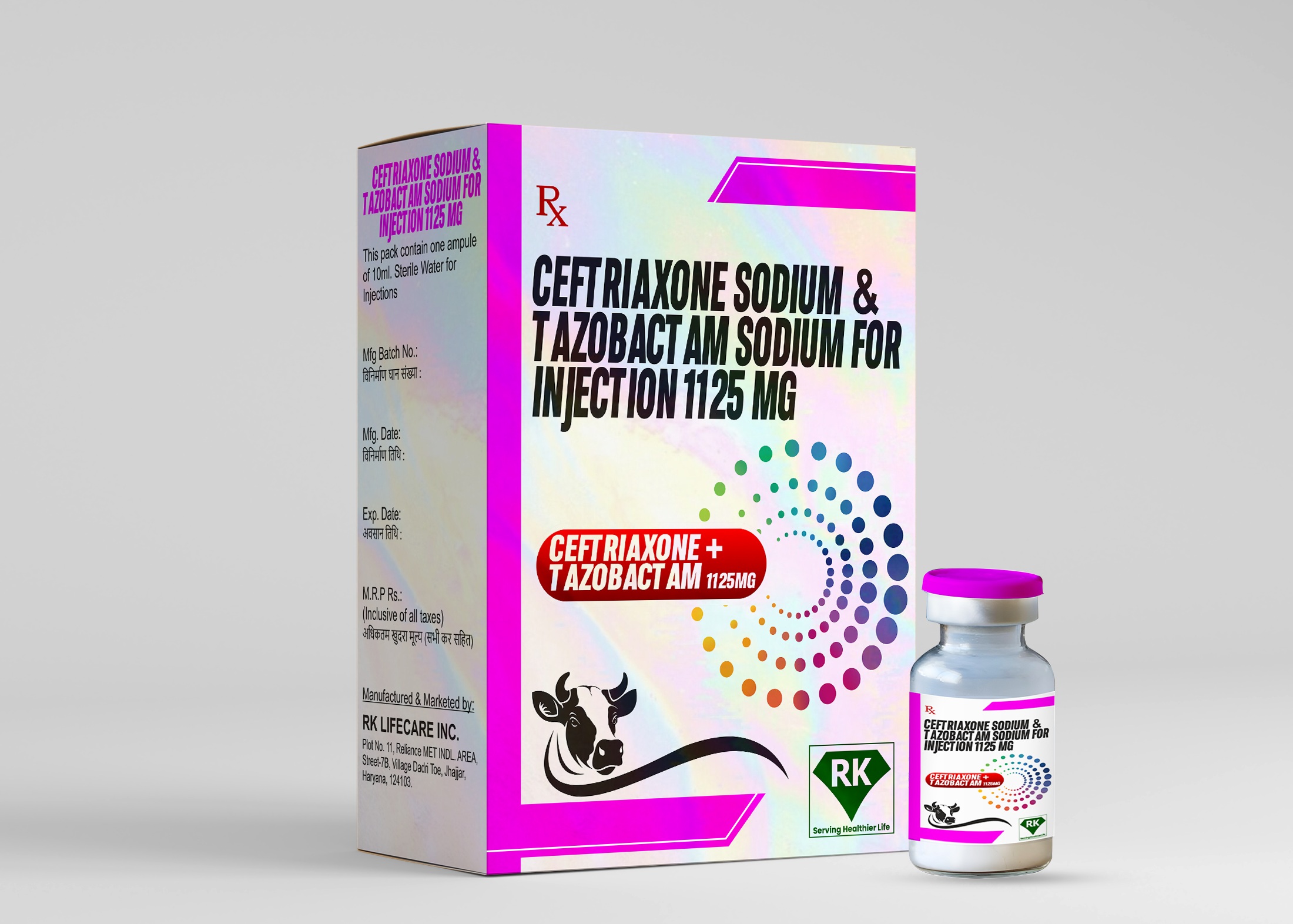
R K Lifecare Inc: Leading Manufacturer and Wholesaler of Ceftriaxone Sodium and Tazobactam Sodium for Injection 1125mg
At R K Lifecare Inc, We are proud to be recognized as the leading manufacturer of Ceftriaxone Sodium and Tazobactam Sodium for Injection 1125mg in India. With a commitment to excellence, we specialize in the production and wholesale distribution of Ceftriaxone Sodium Injection and Tazobactam Sodium Injection to meet the growing demand for effective treatment against serious bacterial infections.
As Ceftriaxone Sodium Injection manufacturers in India, We have established ourselves as a trusted supplier to hospitals, clinics, and healthcare facilities in India and beyond. Ceftriaxone Sodium and Tazobactam Sodium for Injection 1125mg formulations are a vital part of modern healthcare, providing a combination of two powerful antibiotics that work synergistically to fight infections caused by a variety of bacterial pathogens, including those that are resistant to other antibiotics.
As a manufacturer, wholesaler and supplier, our role is vital in ensuring that healthcare providers have consistent access to high-quality Ceftriaxone Sodium and Tazobactam Sodium Injection 1125mg, allowing them to deliver effective treatment to patients who need immediate and reliable care.
The Ceftriaxone Sodium and Tazobactam Sodium Injection 1125mg formulation combines the third-generation cephalosporin antibiotic ceftriaxone with tazobactam, a beta-lactamase inhibitor. This combination has been proven to provide improved effectiveness in treating a wide range of bacterial infections, including respiratory tract, urinary tract, intra-abdominal infections, and skin and soft tissue infections.
At R K Lifecare Inc, We recognize the critical role these injections play in treating life-threatening infections, which is why we ensure that each batch is produced under strict quality control standards.
As a wholesaler of Ceftriaxone Sodium Injection in India, We work tirelessly to ensure that healthcare providers across the country have access to this vital medication in a timely and cost-effective manner. Our distribution network extends to Ceftriaxone Sodium Injection distributors in India, helping us meet the demands of hospitals, pharmacies, and healthcare institutions that rely on our products to treat patients suffering from bacterial infections.
Our role as a Ceftriaxone Sodium Injection supplier in India is not limited to just the production and distribution of the drug. We take great pride in the comprehensive process of manufacturing Ceftriaxone Sodium and Tazobactam Sodium for Injection 1125mg, from the procurement of the initial raw materials to the final distribution of the finished product.
We understand that quality and safety are paramount when it comes to injectable medications, and as such, we adhere to rigorous industry standards and regulatory guidelines to ensure that each vial is safe, effective, and of the highest quality.
This dedication to excellence has earned us a reputation as the leading Ceftriaxone Sodium Injection supplier in Delhi NCR, and we continue to expand our reach, offering our products to Ceftriaxone Sodium Injection dealers and distributors in India and abroad.
At R K Lifecare Inc, We take a customer-centric approach, ensuring that our partners, be they hospitals, clinics or pharmacies, can rely on us for the supply of Ceftriaxone Sodium and Tazobactam Sodium Injection 1125mg whenever they need it.
Our extensive network of Tazobactum Sodium Injection wholesalers in India and Tazobactum Sodium Injection distributors in India helps ensure that our products are readily available to healthcare providers across a wide geographical area. This ability to meet consistent demand has made us a key player in the pharmaceutical industry, trusted by both local and international healthcare providers.
Manufacturing Process of Ceftriaxone Sodium and Tazobactam Sodium Injection 1125mg
The journey of production of Ceftriaxone Sodium and Tazobactam Sodium Injection 1125mg begins with careful procurement of raw materials. At R K Lifecare Inc, We insist on sourcing only the highest quality active pharmaceutical ingredients (APIs) from certified suppliers.
This ensures that the raw materials used in the production of our injectable antibiotics are pure, potent and meet strict regulatory standards. Once the raw materials are procured, they undergo quality control (QC) testing to ensure they meet the required specifications. This testing is a crucial step in preventing contamination and ensuring that only the highest quality ingredients enter the production process.
After the raw materials pass the QC tests, they are carefully blended to create the Ceftriaxone Sodium and Tazobactam Sodium 1125mg Solution for Injection. This step is known as formulation and blending, which requires precision and expertise. Our team of experienced professionals ensures that each ingredient is measured accurately, and the mixing process is carried out in a sterile environment to avoid any contamination.
Once the solution is properly mixed, it undergoes sterile filtration, which removes any potential contaminants and ensures that the final product remains sterile throughout the manufacturing process.
For injectable products like Ceftriaxone Sodium and Tazobactam Sodium 1125mg for Injection, maintaining sterility is of the utmost importance. After sterile filtration, the solution is filled into vials using state-of-the-art equipment designed to minimize the risk of contamination.
Each vial is filled with an accurate dose of Ceftriaxone Sodium and Tazobactam Sodium 1125mg, ensuring that healthcare providers can administer the correct amount of medication to their patients. The vials are then sealed, and the stoppering and sealing process is conducted to maintain the sterility of the product during storage and distribution.
Lyophilization (freeze drying) is used for specific formulations of injectable medications, including Ceftriaxone Sodium and Tazobactam Sodium For Injection 1125mg. The process involves freezing the solution and removing the water content via vacuum, which helps preserve the active ingredients and extend the product's shelf life. Lyophilized formulations are particularly useful when the drug needs to remain stable for long periods of time and under various storage conditions. After lyophilization, the vials are closed and sealed to ensure the final product remains sterile and effective.
Quality Control (QC) and Quality Assurance (QA)
At R K Lifecare INC, We take great pride in our quality control (QC) processes. Every batch of Ceftriaxone Sodium and Tazobactam Sodium for Injection 1125mg is rigorously tested in our in-house laboratories to ensure it meets all safety, strength, and purity requirements. Our quality assurance (QA) team thoroughly inspects the manufacturing process to ensure all procedures are properly followed and each vial of Ceftriaxone Sodium and Tazobactam Sodium for Injection 1125mg is of the highest possible quality. Our commitment to QC and QA ensures that our products are safe, effective, and compliant with regulatory standards.
Uses and Side Effects
The combination of Ceftriaxone Sodium and Tazobactam Sodium for Injection 1125mg is used to treat a wide range of bacterial infections including respiratory tract infections, urinary tract infections, intra-abdominal infections, and skin and soft tissue infections. The combination of the third-generation cephalosporin ceftriaxone and the beta-lactamase inhibitor tazobactam increases the effectiveness of treatment by inhibiting bacterial resistance mechanisms. This makes it a valuable tool in the treatment of severe infections caused by multi-drug resistant pathogens.
However, like all medicines, Ceftriaxone Sodium and Tazobactam Sodium 1125mg for Injection can cause side effects. Common side effects include injection site reactions, nausea, vomiting, and diarrhea. Serious side effects, although rare, can include allergic reactions, such as rash, itching or swelling, and more severe reactions such as anaphylaxis. It is important that healthcare providers monitor patients closely during treatment and be prepared to manage any adverse reactions immediately.
Labeling and Packaging
The labeling and packaging of Ceftriaxone Sodium and Tazobactam Sodium 1125mg for Injection is done very carefully. Each vial is labeled with essential information, including dosage strength, instructions for use, and potential side effects. The packaging is designed to protect the product from contamination and damage during transportation and storage. Additionally, the packaging ensures that healthcare providers have all the necessary information to use the drug safely and effectively.
Storage and Storage
Proper storage and warehousing are essential to maintain the stability and efficacy of Ceftriaxone Sodium & Tazobactam Sodium for Injection 1125mg. At R K Lifecare INC, We ensure that our storage facilities are temperature-controlled and comply with industry standards to protect product integrity. Our storage system allows us to track and manage inventory efficiently, ensuring that we can meet demand in a timely manner. We take great care in storing our products to ensure that they remain effective and safe until they reach healthcare providers.
Distribution and Supply Chain
Our efficient distribution and supply chain network ensures that Ceftriaxone Sodium & Tazobactam Sodium for Injection 1125mg is available to healthcare providers when they need it. We work with a network of Ceftriaxone Sodium Injection distributors in India, Tazobactam Sodium Injection distributors in India, and Ceftriaxone Sodium Injection wholesalers in India to ensure that our products are accessible across the country. We are committed to providing high quality medicines to hospitals, clinics, and pharmacies on time to ensure that patients receive the required treatment without any delay.

22 Jan 2026
20 Jan 2026

19 Jan 2026

13 Jan 2026

08 Jan 2026