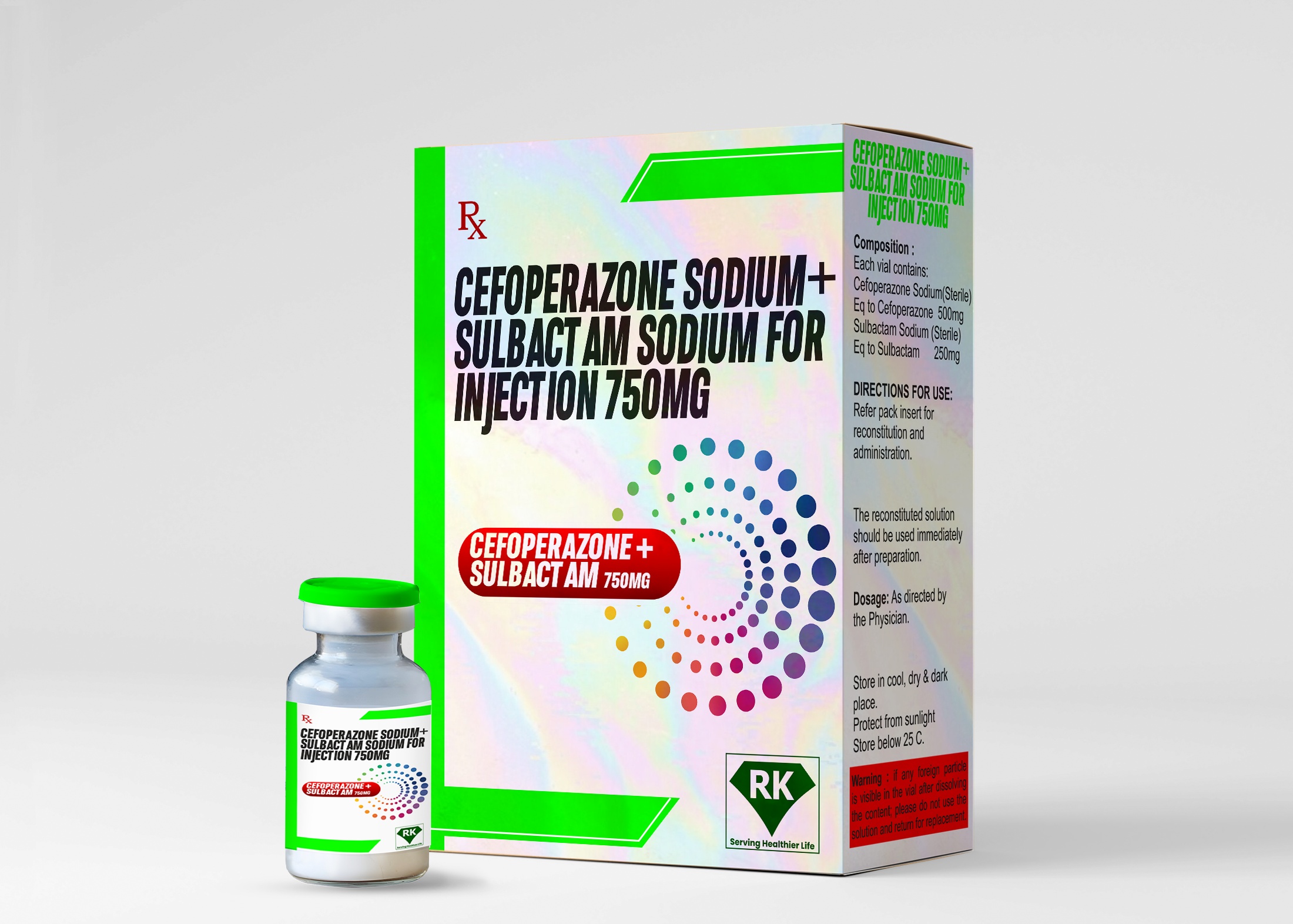
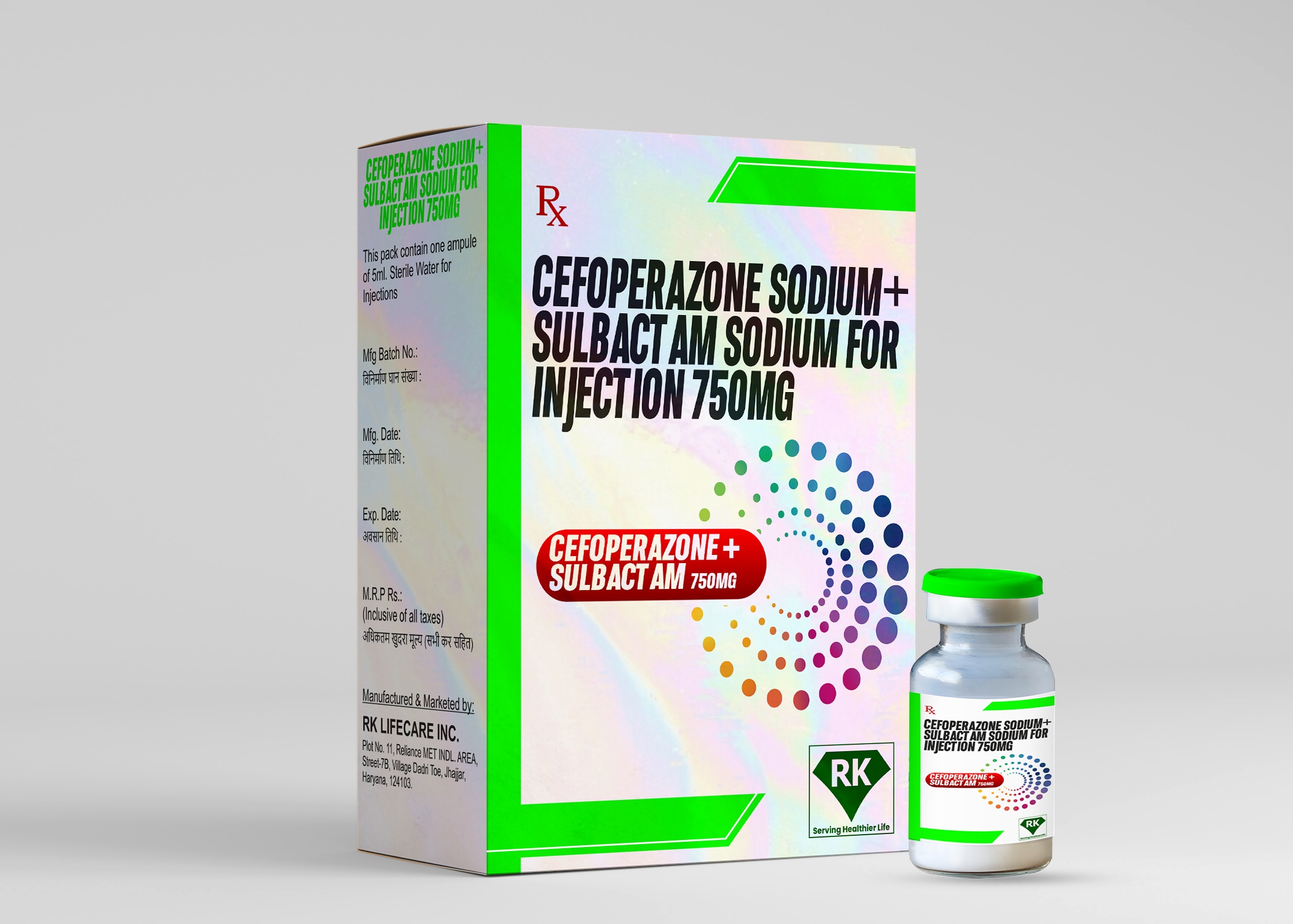
Introduction of Cefoperazone Sodium and Sulbactam Sodium for Injection
Cefoperazone Sodium and Sulbactam Sodium for Injection is a combination drug used to treat a wide range of bacterial infections. This potent antibiotic combination is used in clinical settings to treat infections caused by bacteria that are resistant to many common antibiotics.
The combination of the third-generation cephalosporin cefoperazone and the beta-lactamase inhibitor sulbactam works synergistically to enhance the efficacy of cefoperazone by inhibiting the beta-lactamase enzymes produced by bacteria. This improves the infection-fighting ability of the antibiotic.
In India, the demand for cefoperazone sodium and sulbactam sodium injection is increasing due to their effectiveness in treating severe infections such as pneumonia, sepsis, urinary tract infections, skin and soft tissue infections, and intra-abdominal infections.
As a leading manufacturer of Cefoperazone Sodium and Sulbactam Sodium for Injection 750mg, R K Lifecare INC, Provides a high-quality product that meets the stringent standards of the pharmaceutical industry. The drug is available in sterile injectable form, ensuring safe and reliable administration for patients. At R K Lifecare INC.
We are committed to providing world-class pharmaceutical products and solutions in both domestic and international markets. As one of the most trusted Cefoperazone Sodium manufacturers in India, we focus on delivering products that meet the highest standards of quality and safety.
With years of expertise in the industry, we are proud to be recognized as a leading Cefoperazone Sodium wholesaler, Cefoperazone Sodium distributor and Cefoperazone Sodium supplier in India.
Our products are manufactured using state-of-the-art facilities that follow good manufacturing practice (GMP) and other regulatory guidelines. Our manufacturing process ensures that the final product is of the highest purity and efficacy. This attention to detail has made us a preferred partner for healthcare providers in India and beyond.
Partnerships with Cefoperazone Sodium and Sulbactam Sodium manufacturers ensure that healthcare providers have access to reliable antibiotics that can effectively fight multi-drug resistant bacteria. By choosing R K Lifecare INC, Healthcare professionals are assured of getting the best quality injectable medicines from reliable Cefoperazone Sodium and Sulbactam Sodium wholesalers and distributors.
Our commitment to providing high-end pharmaceutical products makes us a trusted name in the pharmaceutical industry. By catering to the needs of hospitals, clinics, and other healthcare institutions, R K Lifecare INC plays a vital role in the distribution and supply of Cefoperazone Sodium and Sulbactam Sodium in India and beyond.
Whether you are a distributor, wholesaler, or healthcare provider, our focus on quality and timely delivery makes us the preferred Cefoperazone Sodium supplier.
Uses and Side Effects
Uses: Cefoperazone Sodium and Sulbactam Sodium Injection 750mg is used to treat various infections caused by bacteria. It is commonly prescribed for patients with pneumonia, urinary tract infections, soft tissue infections, intra-abdominal infections, and sepsis. Combination therapy increases the effectiveness of cefoperazone by preventing bacterial resistance. This drug is often used both in hospital and outpatient settings to manage infections in critically ill patients.
Side Effects: Like any drug, cefoperazone sodium and sulbactam sodium can cause side effects. Some common side effects include gastrointestinal problems such as nausea, vomiting, and diarrhea. In rare cases, patients may experience allergic reactions, skin rashes, or liver function abnormalities. It is important for patients to consult their healthcare provider to monitor for any adverse reactions during treatment.
Labeling and Packaging
Cefoperazone Sodium and Sulbactam Sodium Injection 750mg is available in sterile vials, which ensures its safety and integrity during distribution and storage. The product's labeling includes all the necessary information about its use, dosage instructions, possible side effects, and storage requirements. The packaging is designed to protect the drug from external factors such as light and moisture, which can degrade its quality. The vials are securely sealed, and the packaging conforms to international drug regulations to ensure the safety of the product.
Storage and Warehousing
To maintain the efficacy and stability of Cefoperazone Sodium and Sulbactam Sodium for Injection, it must be stored under proper conditions. The product should be kept in a cool and dry place, away from direct sunlight, at the temperature recommended by the manufacturer. Proper storage and warehousing conditions are important to ensure that the product remains in optimal condition before it reaches healthcare providers.
Distribution and Supply Chain
R K Lifecare INC has an efficient and strong distribution network that ensures timely delivery of Cefoperazone Sodium and Sulbactam Sodium to various regions in India and abroad. Our distribution team works closely with Cefoperazone Sodium wholesalers and distributors to ensure that healthcare providers receive the product on time. We use a reliable supply chain management system that helps us track inventory, reduce delivery times, and minimize disruptions in the availability of this lifesaving antibiotic.
Procurement of Raw Materials
The quality of the raw materials used in the manufacture of Cefoperazone Sodium and Sulbactam Sodium is critical to ensuring the safety and efficacy of the final product. At R K Lifecare INC, We source raw materials from trusted suppliers that meet our strict quality standards. These suppliers are selected based on their ability to consistently deliver high-quality pharmaceutical-grade ingredients that meet regulatory requirements for drug production.
Quality Control (QC) of Raw Materials
Before production begins, all raw materials undergo an extensive quality control process. This includes testing for purity, potency, and absence of contaminants. Our in-house QC team works closely with the procurement team to ensure that only the highest quality ingredients are used in the manufacturing process. Regular audits and inspections are conducted to maintain compliance with the latest pharmaceutical industry standards.
Formulation and Blending
The formulation and blending process for Cefoperazone Sodium and Sulbactam Sodium for Injection involves precise measurement and mixing of the active pharmaceutical ingredients (APIs) in a sterile environment. This step is critical to ensure that the final product delivers the correct dosage and maintains its potency. Our experienced manufacturing team follows stringent protocols to ensure consistency and uniformity in the mixing process.
Sterile Filtration and Filling into Vials
After the manufacturing process, the product undergoes sterile filtration to remove any particles or impurities. The sterile filtration process ensures that the injectable drug is free from contaminants that may affect its safety or efficacy. Once the product is filtered, it is filled into sterile vials under controlled conditions, minimizing the risk of contamination during the filling process.
Lyophilization (Freeze Drying) (For lyophilized injections only)
For lyophilized injections, the product undergoes lyophilization (freeze-drying) to ensure long-term stability. This process removes water from the product, allowing it to be stored in a more stable form. Lyophilization is especially important for injectable antibiotics that are sensitive to degradation in liquid form. Once lyophilized, the product can be reconstituted by healthcare providers before administering it to patients.
Stoppering and Sealing
After filling the drug into vials, the next step is stoppering and sealing. This process ensures that the vials are airtight and protected from contamination, which is important for maintaining the sterility and stability of the product. The vials are sealed using high-quality rubber stoppers and aluminum seals to provide an additional layer of protection from external factors.
Quality Control (QC) and Quality Assurance (QA) Testing
At R K Lifecare INC, We place great emphasis on quality control and quality assurance throughout the manufacturing process. Our QC and QA teams conduct rigorous testing to ensure that every batch of Cefoperazone Sodium and Sulbactam Sodium for Injection meets the required standards for purity, potency, and sterility. These tests are performed at various stages of production to ensure that the final product is safe and effective for patient use.
08 Dec 2025
03 Dec 2025

02 Dec 2025

31 Oct 2025

30 Oct 2025