
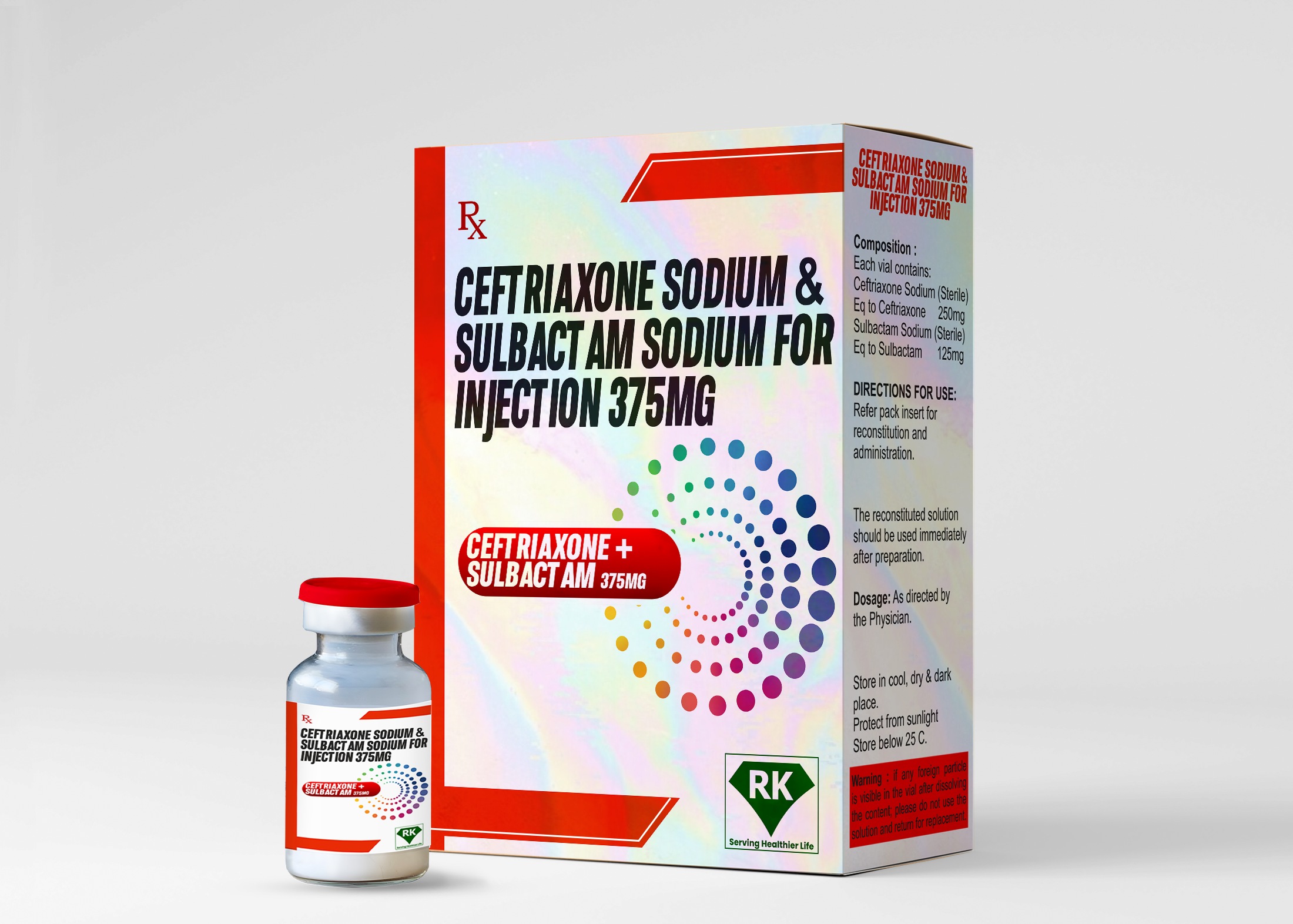
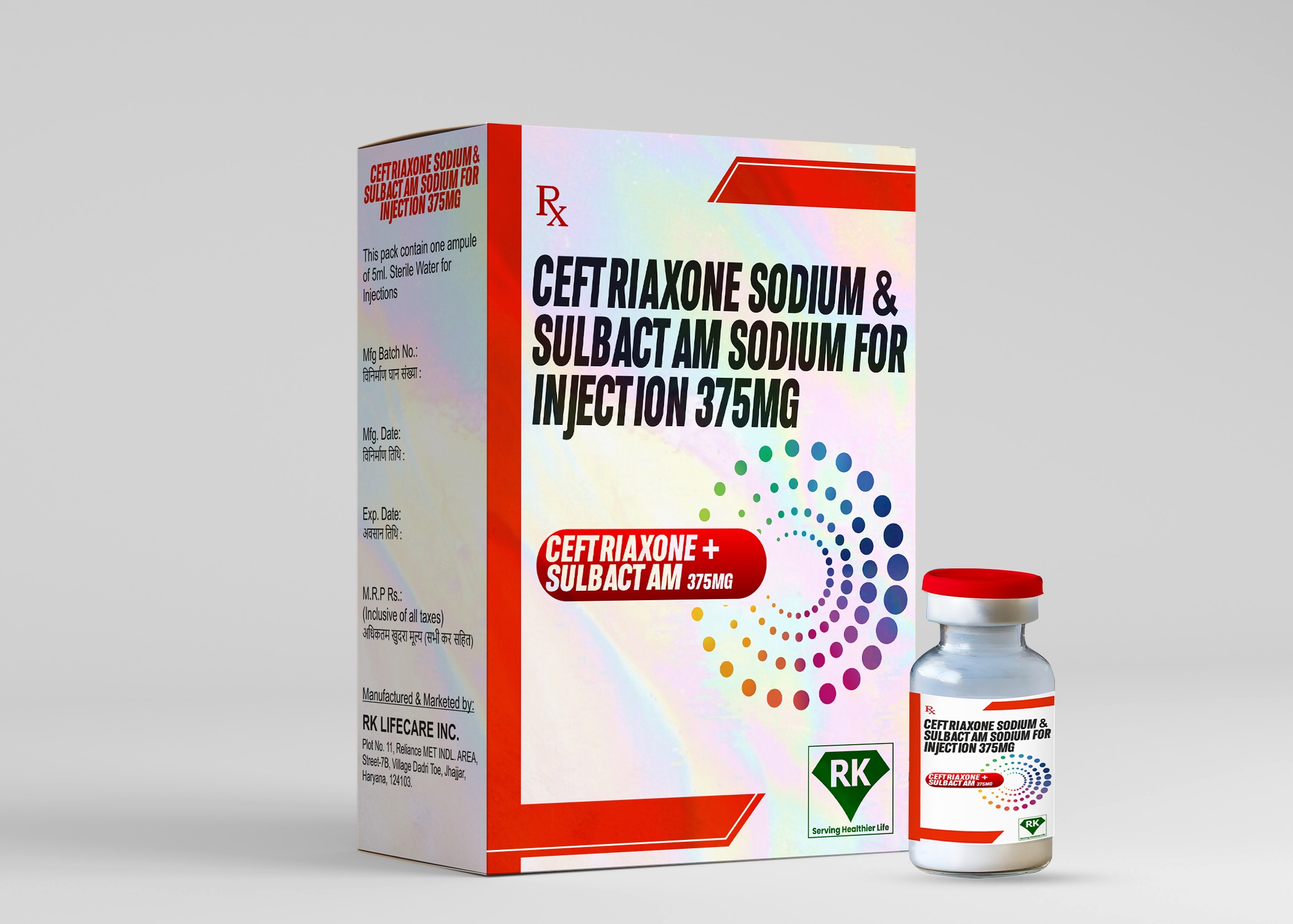
Ceftriaxone Sodium & Sulbactam Sodium for Injection 375mg
At R K Lifecare Inc, we take immense pride in being recognized as the leading Ceftriaxone Sodium manufacturers in India, specializing in the production, wholesale distribution and supply of high-quality injectable antibiotics including Ceftriaxone Sodium and Sulbactam Sodium 375mg for Injection. Based in Delhi, India, we are committed to delivering world-class pharmaceutical solutions, especially in the field of antimicrobial treatment. As Ceftriaxone Sodium suppliers and Sulbactam Sodium Injection wholesalers, we understand the critical role these antibiotics play in the effective management of bacterial infections, and we work tirelessly to ensure that they are accessible to healthcare providers in India and beyond. Our dedication to excellence is reflected in our ability to manufacture pharmaceutical products that not only meet but also exceed the stringent standards set by international regulatory bodies.
Ceftriaxone Sodium is a broad-spectrum cephalosporin antibiotic that is highly effective in treating a variety of severe bacterial infections, including those caused by gram-negative organisms. When it is combined with the powerful beta-lactamase inhibitor, Sulbactam Sodium, the efficacy of Ceftriaxone Sodium is increased, making it even more effective against resistant strains of bacteria that produce beta-lactamase. As reliable Ceftriaxone Sodium wholesalers and Sulbactam Sodium injection distributors, we ensure that our products are not only of the highest quality but are also available in sufficient quantities to meet the growing demand in the healthcare sector.
In India, the increasing prevalence of antibiotic-resistant infections has made the availability of high-quality Ceftriaxone Sodium and Sulbactam Sodium 375mg for Injection more important than ever. These antibiotics are integral to treating infections such as pneumonia, meningitis, urinary tract infections, and bloodstream infections, which are often caused by resistant bacteria. The combination of Ceftriaxone Sodium and Sulbactam Sodium provides a powerful solution to these challenges, as it not only targets bacteria, but also counteracts the enzymes they produce to resist treatment.
As Ceftriaxone Sodium manufacturers in India, we focus on manufacturing these antibiotics with the utmost precision, ensuring that the final product is both effective and safe for use in a wide range of clinical settings. Sulbactam Sodium plays a key role in enhancing the therapeutic benefits of Ceftriaxone Sodium, making it a vital tool for healthcare providers dealing with resistant infections. At R K Lifecare Inc, we are proud to be recognized as the leading Sulbactam Sodium Injection manufacturers in India and are committed to ensuring that our products are available to healthcare providers in India and beyond.
Our manufacturing facility in Delhi follows good manufacturing practices (GMP) and uses state-of-the-art technology to produce Ceftriaxone Sodium and Sulbactam Sodium for Injection 375mg that meets the highest quality standards. As a Ceftriaxone Sodium distributor and Sulbactam Sodium Injection distributor, we have established an extensive supply chain that ensures timely delivery of these essential medicines to hospitals, clinics and pharmacies across the country. Our extensive distribution network allows us to meet the growing demand for Ceftriaxone Sodium and Sulbactam Sodium while maintaining product integrity and quality. Whether you are looking for a reliable Ceftriaxone Sodium wholesaler or Sulbactam Sodium Injection supplier, R K Lifecare Inc is your trusted partner in ensuring access to essential medicines for effective infection management.
Distribution and Supply Chain Process
The distribution and supply of Ceftriaxone Sodium and Sulbactam Sodium Injection 375mg follows a carefully structured process that ensures both efficiency and reliability of product availability. At R K Lifecare Inc, we understand that timely and safe delivery is critical in healthcare, where delays can have serious consequences on patient care. Our distribution network is designed to guarantee that Ceftriaxone Sodium and Sulbactam Sodium Injection reaches healthcare facilities promptly and without compromising on product quality. Our highly trained logistics team works closely with distributors and partners across the country to ensure seamless delivery from our manufacturing facility in Delhi to hospitals, clinics and pharmacies across the country. By maintaining a robust supply chain, we ensure that our products are available wherever they are needed the most.
Procurement of Raw Materials
The quality of any pharmaceutical product begins with the raw materials used in its production. At R K Lifecare Inc, we work with trusted suppliers who provide high-quality Ceftriaxone Sodium and Sulbactam Sodium raw materials. Each batch of raw materials undergoes rigorous testing to ensure that it meets the required standards for identity, purity, and potency. As a Ceftriaxone Sodium manufacturer and Sulbactam Sodium Injection manufacturer, we understand the critical importance of sourcing the best raw materials to guarantee the quality and effectiveness of the final product. Our strict quality control process ensures that only the highest quality ingredients are used in the manufacture of Ceftriaxone Sodium and Sulbactam Sodium for Injection 375mg.
Quality Control (QC) of Raw Materials
Before any raw materials are used in the manufacturing process, they undergo an extensive quality control (QC) assessment. This includes chemical analysis, microbiological testing, and stability testing to verify that ingredients meet our stringent quality standards. As a Sulbactam Sodium Injection supplier and Ceftriaxone Sodium wholesaler, we recognize the critical role quality raw materials play in the success of the final product. We work closely with our suppliers to ensure that raw materials are obtained from reliable sources and are consistently of the highest quality. Our QC team thoroughly inspects each raw material batch before it enters the production process, ensuring that only the best materials are used in the manufacture of Ceftriaxone Sodium and Sulbactam Sodium for Injection 375mg.
Formulation and Blending
Once the raw materials pass QC testing, they are blended in precise proportions according to our formulation guidelines. At R K Lifecare Inc, we use advanced formulation techniques to ensure uniform distribution of active ingredients in each batch. The mixing process is done under carefully controlled conditions to prevent contamination and maintain the integrity of the ingredients. As a Ceftriaxone Sodium manufacturer and Sulbactam Sodium Injection manufacturer, we pay close attention to every detail during the manufacturing process to ensure that the final product is stable, effective, and safe for injection.
Sterile Filtration and Filling into Vials
After mixing, the solution undergoes sterile filtration to remove any potential contaminants. The filtration process ensures that the final product is free from microorganisms and other impurities. After being filtered, the solution is filled into vials under aseptic conditions to maintain its sterility. The vials are carefully filled with the correct dosage of Ceftriaxone Sodium and Sulbactam Sodium 375mg for Injection, ensuring that each vial contains the exact amount needed for effective treatment. The filling process is performed using state-of-the-art equipment that minimizes the risk of human error and ensures consistency across all vials.
Lyophilization (Freeze Drying)
For lyophilized products, the next step is lyophilization (freeze drying). This process removes water from the injectable solution, which helps to extend the product's shelf life and maintain its stability. Lyophilization is a critical step for injectable antibiotics such as ceftriaxone sodium and sulbactam sodium, as it helps to maintain the drug's potency while ensuring it can be stored and transported safely. The lyophilization process is carefully controlled to ensure the final product maintains its effectiveness and safety.
Stoppering and Sealing
Once Ceftriaxone Sodium and Sulbactam Sodium Injection 375mg is lyophilized, the vials are stoppered and sealed to maintain sterility and protect the product from contamination. This step is necessary to ensure that the medication remains safe and effective until it is used. The sealing process is performed in a controlled environment to prevent exposure to external contaminants.
Quality Control (QC) and Quality Assurance (QA) Testing
Before the product is released for distribution, it undergoes a series of quality control (QC) and quality assurance (QA) tests. These tests include sterility testing, potency testing, and stability testing to ensure that Ceftriaxone Sodium and Sulbactam Sodium for Injection 375mg meets all required specifications. As Ceftriaxone Sodium suppliers and Sulbactam Sodium injection distributors, we understand the importance of rigorous testing to ensure that our products are both effective and safe for use in clinical settings.
Uses and Side Effects
Ceftriaxone Sodium is a broad-spectrum antibiotic used to treat a wide variety of infections, including respiratory tract infections, urinary tract infections, and bloodstream infections. When it is combined with sulbactam sodium, the effectiveness of ceftriaxone sodium is increased, making it more effective against beta-lactamase-producing organisms that are otherwise resistant to treatment. However, like all medications, Ceftriaxone Sodium and Sulbactam Sodium Injection 375mg may cause more serious side effects such as nausea, diarrhea, allergic reactions, and, in rare cases, anaphylaxis or liver toxicity. Healthcare providers should carefully monitor patients for any adverse reactions.
Labeling and Packaging
Each vial of Ceftriaxone Sodium and Sulbactam Sodium Injection 375mg is labeled with essential information, including dosing instructions, storage recommendations, and potential side effects. Our labeling process ensures that healthcare providers have all the necessary information to administer the medication safely. Packaging is designed to protect the product from contamination and maintain its sterility during transportation and storage.
Storage and Warehousing
Proper storage is crucial to maintaining the quality and potency of Ceftriaxone Sodium and Sulbactam Sodium Injection 375mg. At R K Lifecare Inc, we follow strict storage guidelines to ensure that our products are stored in a temperature-controlled environment and protected from light and moisture. Our warehouse is equipped with advanced systems to monitor and control storage conditions, ensuring that our products remain safe and effective until they reach healthcare providers.
In conclusion, R K Lifecare Inc. is a trusted name in the manufacturing and wholesale distribution of Ceftriaxone Sodium and Sulbactam Sodium Injection 375mg in India. Through our commitment to quality, innovation, and customer satisfaction, we have established ourselves as a leading provider of high-quality antibiotics. As Ceftriaxone Sodium manufacturers and Sulbactam Sodium Injection suppliers, we are dedicated to ensuring that our products reach healthcare providers across India, allowing them to provide the best possible care to their patients.



.jpg)
.jpg)

.jpg)
 (1).jpg)
.jpg)




