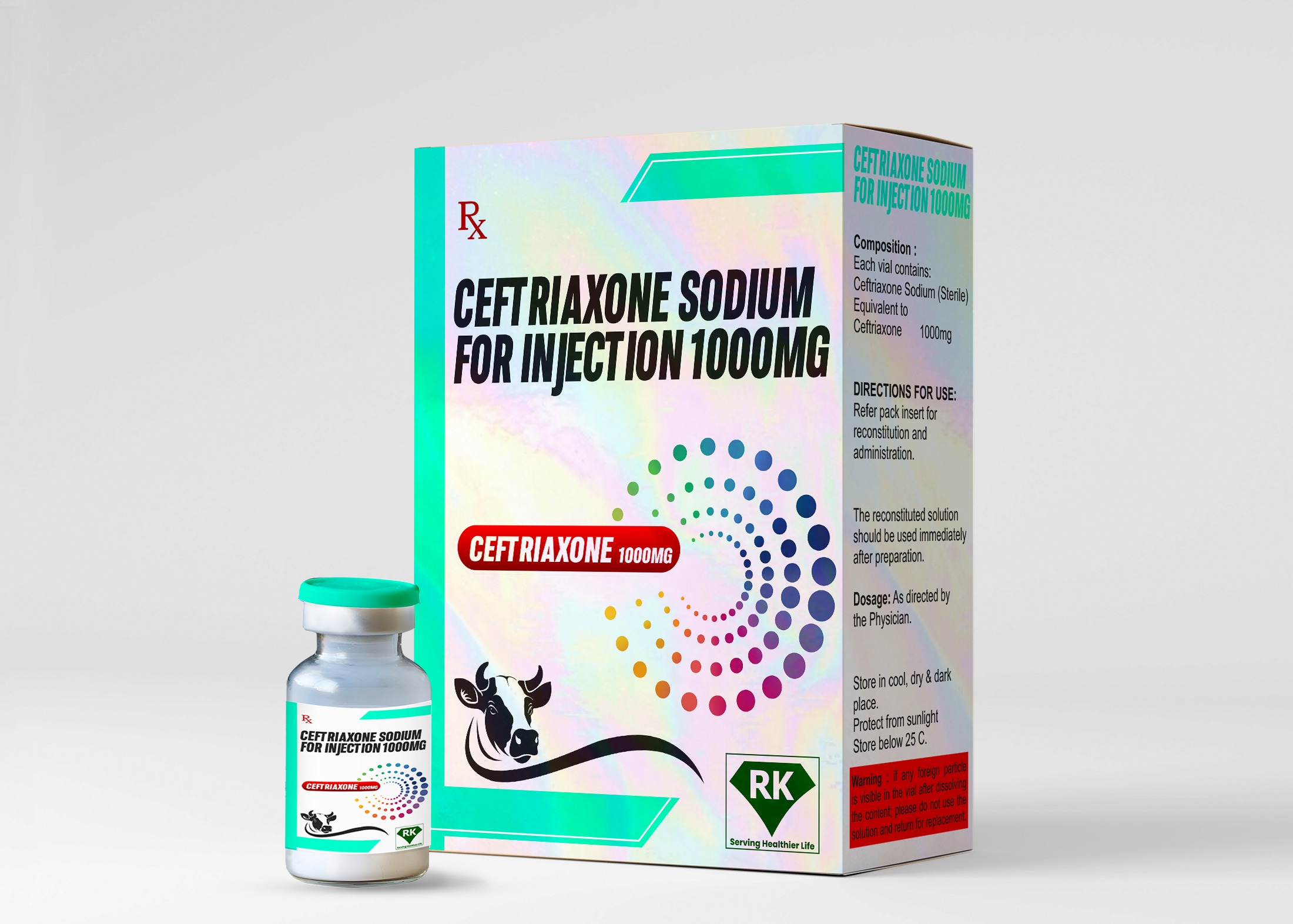
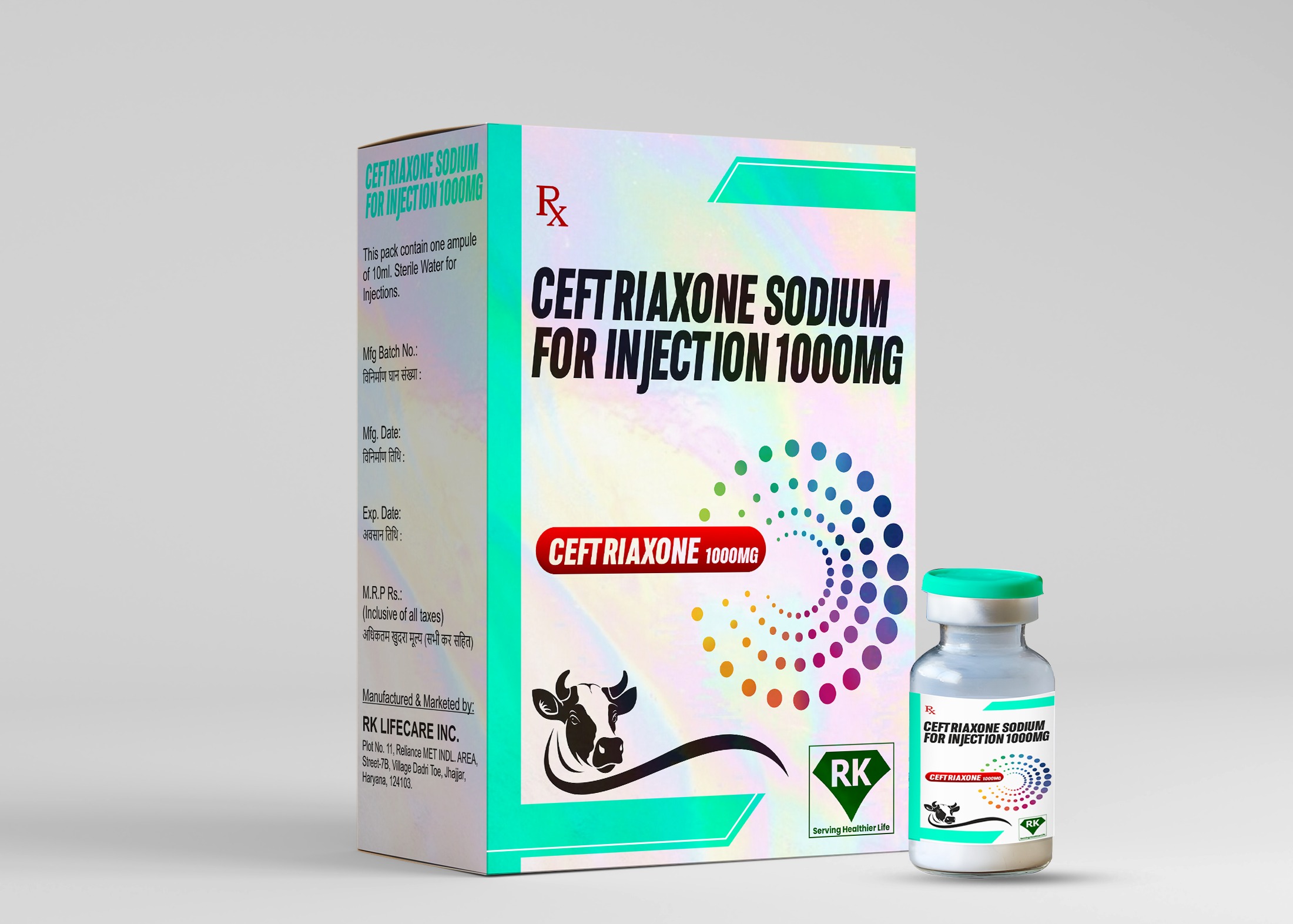
At R K LIFECARE INC, We take great pride in being one of the leading Ceftriaxone Sodium Injection 1000 mg manufacturers in India, committed to providing high-quality pharmaceutical products that contribute to better health outcomes.
As a reliable Ceftriaxone Sodium Injection wholesaler in India, we have established ourselves as a go-to supplier for healthcare facilities in need of this life-saving drug. Ceftriaxone Sodium Injection 1000 mg is an essential antibiotic used in the treatment of a wide range of serious bacterial infections.
This drug is particularly important in the management of infections of the lungs, blood, urinary tract, meninges, and skin. Our company serves as a reliable Ceftriaxone Sodium Injection supplier in India Focusing on strict adherence to quality standards.
We also serve as Ceftriaxone Sodium Injection distributors in India, which helps us ensure that our products are available in various regions, including Ceftriaxone Sodium Injection in Delhi NCR and beyond. Whether you require bulk supplies or individual vials, R K Lifecare INC is committed to offering the highest quality Ceftriaxone Sodium Injection.
The role of Ceftriaxone Sodium Injection manufacturer in India is crucial in today's healthcare landscape. Infections continue to be a significant threat to public health, and Ceftriaxone, with its broad-spectrum activity against a variety of bacterial pathogens, is a vital tool in the treatment and management of these conditions.
Our commitment to quality manufacturing, supported by state-of-the-art facilities and rigorous testing protocols, allows us to consistently produce an antibiotic that meets global standards of safety, efficacy, and reliability.
As part of our responsibility as Ceftriaxone Sodium Injection distributors, We ensure that distribution channels are streamlined to guarantee fast and reliable deliveries to all our partners. By focusing on efficient distribution, we enable healthcare providers to access Ceftriaxone Sodium Injection dealers who can assist them in meeting their pharmaceutical needs without delay.
We feel proud to be able to provide Ceftriaxone Sodium Injection in Delhi NCR, one of the most important regions for healthcare in India. As Ceftriaxone Sodium Injection suppliers, we provide solutions for all types of healthcare settings, ensuring that those in need get the treatment they deserve.
As Ceftriaxone Sodium Injection manufacturers in India, R K Lifecare INC is dedicated to maintaining the highest levels of excellence at every stage of the manufacturing process. From sourcing raw materials to final product delivery, we emphasize quality control, regulatory compliance, and innovative production techniques to ensure the integrity of the final product.
Our production of Ceftriaxone Sodium 1000 mg for Injection is carried out under stringent quality assurance measures that meet both national and international guidelines. This ensures that the product you get is not only safe but also highly effective in treating bacterial infections.
In this article, we will take a closer look at the extensive steps involved in the production process, from the procurement of raw materials to the delivery of the final product, as well as how these elements come together to create an antibiotic that healthcare professionals can rely on.
Sterile Filtration and Filling into Vials
The process of manufacturing Ceftriaxone Sodium for Injection 1000 mg begins with the essential practice of sterile filtration. As an injectable drug, sterility is paramount to ensure patient safety. At R K Lifecare INC, We use advanced sterile filtration techniques to eliminate any microbial contamination that could potentially compromise the safety and efficacy of the final product. The filtration process is performed under aseptic conditions to ensure that the Ceftriaxone Sodium for Injection 1000 mg solution is free from any harmful pathogens.
Once filtration is complete, the finished solution is filled into vials that are specifically designed to store and preserve the integrity of the drug. This step is critical because the exact dose of the active pharmaceutical ingredient (API) must be accurately delivered into each vial.
Lyophilization (Freeze Drying)
For certain formulations, including Ceftriaxone Sodium for Injection, the process of lyophilization (freeze-drying) is used to increase the stability and shelf life of the drug. Lyophilization involves freezing the drug solution and removing the water content under vacuum. This process ensures that the drug remains stable and retains its potency for a long time.
After lyophilization is complete, Ceftriaxone Sodium Injection 1000 mg is in a lyophilized (freeze-dried) form, which can be reconstituted with the appropriate diluent before use. The freeze-dried form not only increases the shelf life of the drug but also makes transportation and storage easier without compromising the quality of the product.
Stoppering and Sealing
After Ceftriaxone Sodium for Injection 1000 mg has been filled into vials and undergone the lyophilization process (if applicable), the next critical step is stopping and sealing the vials. This ensures that the contents remain protected from contamination and environmental factors.
At R K Lifecare INC, We use high-quality stoppers that fit securely to prevent air and moisture from compromising the consistency of the injection. The sealing process is done using automated machines that ensure uniformity and accuracy. Each vial is carefully inspected to ensure that the stopper is correctly placed and securely sealed before moving on to the next step in the production process.
Quality Control (QC) and Quality Assurance (QA) Testing
As a Ceftriaxone Sodium Injection manufacturer in India, we emphasize on both quality control (QC) and quality assurance (QA). Each batch of Ceftriaxone Sodium 1000 mg for Injection undergoes a series of rigorous tests to ensure that it meets the highest standards of quality.
This includes testing for sterility, potency, pH level, and stability. Our QC team is responsible for evaluating the physical, chemical, and microbiological properties of the product, ensuring that the final formulation adheres to the strictest safety standards. In addition, our QA team reviews every step of the manufacturing process to ensure that it complies with regulatory requirements and industry best practices. Through these extensive QC and QA processes, we ensure that the Ceftriaxone Sodium 1000 mg for Injection produced at R K Lifecare INC is safe, effective, and of the highest quality.
Uses and Side Effects
Ceftriaxone Sodium 1000 mg for Injection is used to treat a broad spectrum of bacterial infections. It is effective in treating infections caused by sensitive bacteria, including respiratory tract infections (such as pneumonia), urinary tract infections, meningitis, and blood infections. It works by stopping the growth of bacteria, thereby helping to eliminate the infection from the body. However, like all medicines, Ceftriaxone Sodium for Injection can cause side effects.
The most common side effects include pain or irritation at the injection site, allergic reactions, diarrhea, rash, and fever. In some rare cases, serious side effects such as severe allergic reactions, liver dysfunction, or kidney problems may occur. It is important that health care professionals monitor patients closely for any adverse reactions.
Labeling and Packaging
Proper labeling and packaging of Ceftriaxone Sodium for Injection 1000 mg is essential to ensure proper use and safety. At R K Lifecare INC, We ensure that each vial of Ceftriaxone Sodium for Injection is labeled with important information, including dosage, storage instructions, batch number, and expiration date. The packaging is designed to protect the product from environmental factors such as humidity and temperature fluctuations during transportation. Our packaging meets both national and international regulatory standards, ensuring that the product remains intact and fully functional until it reaches the end user.
Storage and Warehouse
Storage of Ceftriaxone Sodium for Injection 1000 mg requires careful attention to temperature and humidity control. At R K Lifecare INC, We maintain state-of-the-art warehouse facilities that adhere to stringent storage conditions. The temperature-controlled environment ensures that the product remains stable and effective throughout its shelf life. Our inventory management system tracks product quantities, expiration dates, and batch numbers to ensure only the freshest stock is shipped to our customers.
Distribution and Supply Chain As a Ceftriaxone Sodium Injection supplier in India, we have developed a robust distribution and supply chain process to ensure our products are efficiently delivered to healthcare providers. We leverage an extensive network of distribution channels to provide timely deliveries of Ceftriaxone Sodium for Injection to hospitals, clinics, and pharmacies across India. Our streamlined supply chain ensures our customers receive their orders on time and in excellent condition, with minimal delays.
This efficient distribution network is the key to maintaining a reliable and uninterrupted supply of this critical medicine. Procurement of Raw Materials The production of Ceftriaxone Sodium 1000mg for Injection begins with the procurement of high-quality raw materials. We source our active pharmaceutical ingredients (APIs) and excipients from trusted suppliers who meet our stringent quality standards. These raw materials are essential to ensure that the final product is effective and safe for use. We also ensure that suppliers comply with regulatory requirements to maintain the quality and consistency of the raw materials used in production. Quality Control (QC) of Raw Materials
Before any raw materials are used in the production process, they undergo rigorous quality control tests. These tests assess the purity, potency, and safety of the ingredients to ensure they meet our high standards. Our QC team conducts thorough testing on all raw materials to ensure they are free from contaminants and impurities that could compromise the quality of the final product.
Formulation and Blending
Once the raw materials pass the necessary quality control checks, they are blended to form the formulation for Ceftriaxone Sodium for Injection 1000mg. The blending process ensures that the active ingredient is evenly distributed throughout the solution, providing a consistent dosage in every vial. The formulation is then carefully tested to ensure it meets the required potency, stability, and quality specifications.

12 Feb 2026

09 Feb 2026

07 Feb 2026
.jpg)
06 Feb 2026

04 Feb 2026