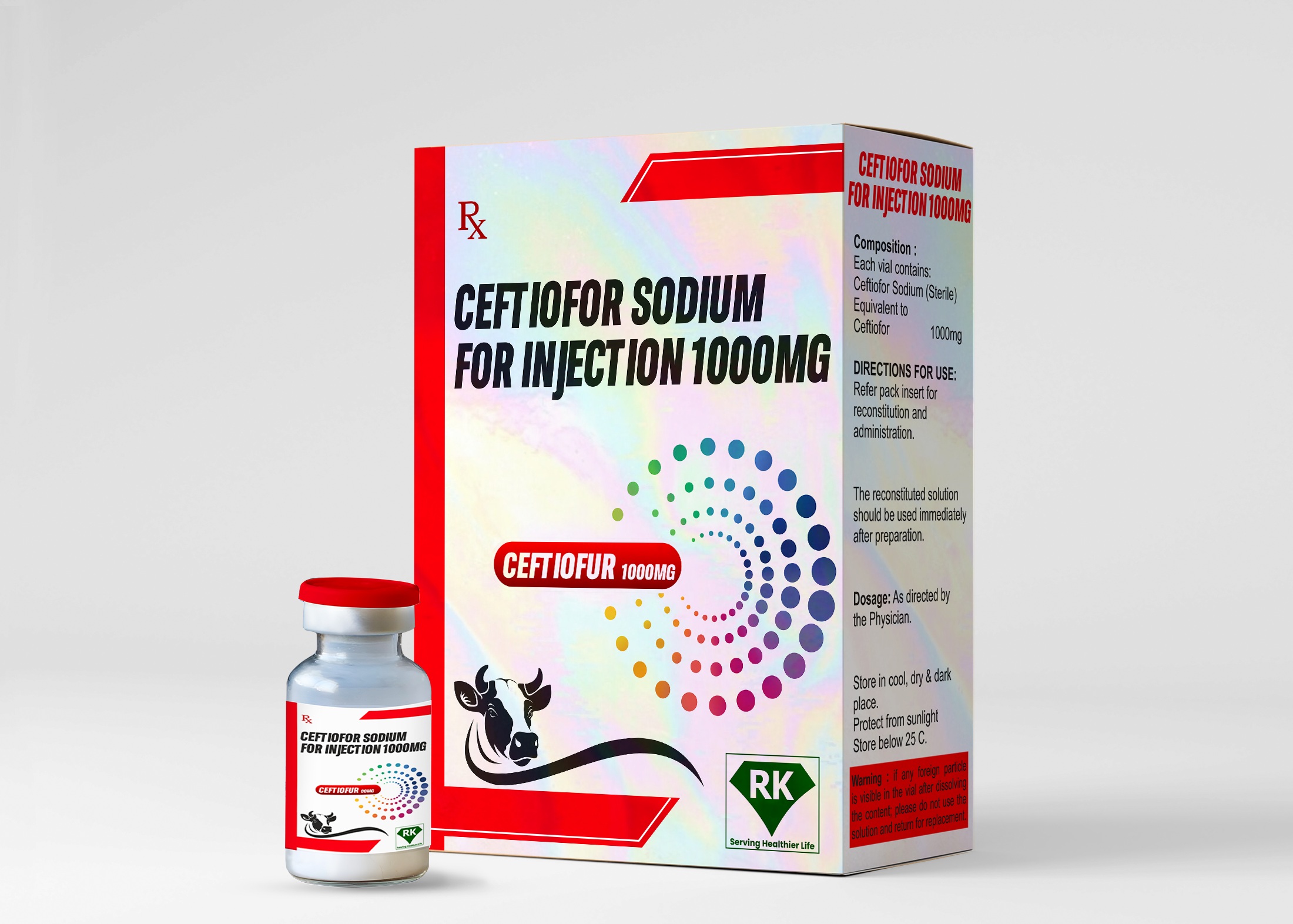
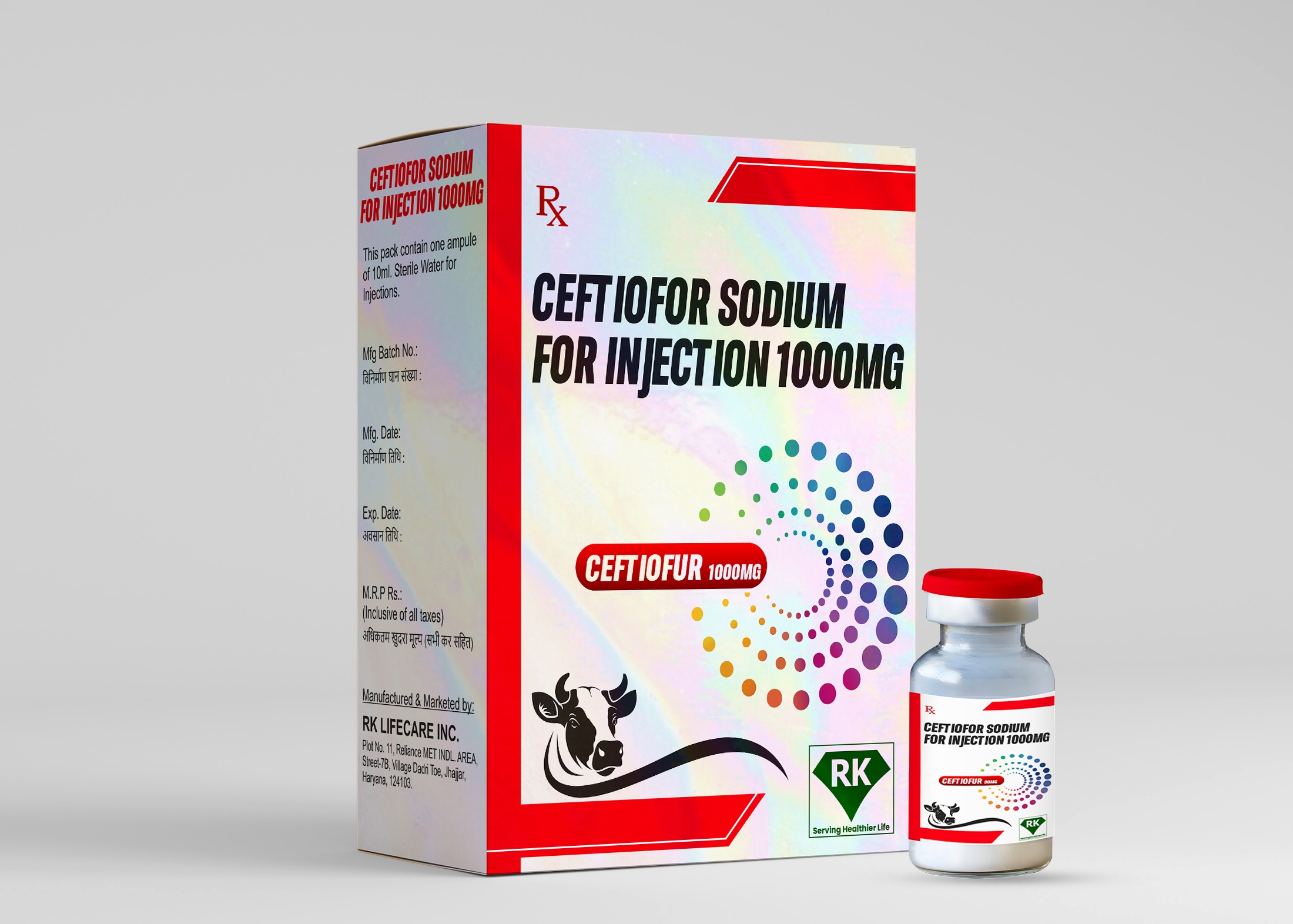
At R K Lifecare INC, We take great pride in our role as Ceftiofur Sodium Injection manufacturers in India, offering a range of high-quality pharmaceutical solutions that meet the ever-evolving needs of the healthcare industry.
As a leading player in the manufacturing and wholesale business of Ceftiofur Sodium Injection 1000mg in India, we have established ourselves as a trusted name in the pharmaceutical sector. Our focus is on manufacturing potent, reliable and effective injections that meet international standards of safety and efficacy, ensuring the best outcomes for patients undergoing treatment.
Ceftiofur Sodium is an advanced third-generation cephalosporin antibiotic, widely recognized for its broad-spectrum antimicrobial activity, especially in the treatment of various bacterial infections. As a wholesaler of Ceftiofur Sodium Injection in India, we ensure the availability of our products to a wide range of healthcare professionals and distributors, ensuring that they are easily accessible across India, including critical areas such as Ceftiofur Sodium Injection in Delhi NCR.
Our manufacturing process is built on a foundation of excellence, combining advanced technologies with strict adherence to regulatory standards. We work diligently as Ceftiofur Sodium Injection distributors in India and Ceftiofur Sodium Injection suppliers in India to bridge the gap between production and healthcare providers, ensuring that medical institutions and pharmacies have access to high-quality injections.
Our experienced team handles every stage of production with precision – from raw material procurement and manufacturing to packaging and distribution – guaranteeing that every vial of Ceftiofur Sodium Injection meets the highest standards of quality and reliability. With an ever-growing presence in the market, we are dedicated to becoming the leading Ceftiofur Sodium Injection supplier in India and contributing significantly to improving patient health outcomes.
Manufacturing Process of Ceftiofur Sodium 1000mg for Injection
At R K Lifecare INC, We prioritize efficiency and precision at every stage of our Ceftiofur Sodium Injection manufacturing process to create a final product that adheres to the highest quality standards. Below is an overview of the critical steps in our production process, all of which are conducted in compliance with international good manufacturing practice (GMP) and rigorous internal standards to ensure the efficacy, safety, and consistency of each batch of Ceftiofur Sodium Injection.
Procurement of Raw Materials
The journey of producing Ceftiofur Sodium Injection 1000mg in India begins with the procurement of high-quality raw materials. At R K Lifecare INC, We source the necessary chemical compounds and ingredients from trusted suppliers that meet our stringent quality requirements. All ingredients, including Ceftiofur Sodium, undergo intensive testing for purity and authenticity before they are approved for use in production. This ensures that the base for each batch of Ceftiofur Sodium Injection is of the highest quality, which is essential to maintaining the integrity of the final product. We take great care in selecting only suppliers who comply with industry standards and regulatory requirements, ensuring that product quality and safety are never compromised.
Quality Control (QC) of Raw Materials
Once the raw materials are received, they undergo extensive quality control (QC) testing. These tests include confirming the identity, potency, and purity of the ingredients. Only ingredients that pass these rigorous QC checks are used in the production of Ceftiofur Sodium Injection 1000mg. Our dedicated QC team is responsible for ensuring that all ingredients comply with established specifications, and that the products are safe for human and veterinary use. This quality control step is essential to eliminate contaminants and ensure that no substandard ingredients reach the manufacturing stage. As a result, we can confidently supply high quality Ceftiofur Sodium Injection to the market.
Formulation and Blending
The next step in our manufacturing process is the formulation and blending of the active pharmaceutical ingredients. In this step, Ceftiofur Sodium is mixed with other excipients to form a precise injectable solution. Formulation is a critical process that requires careful calculations to ensure that each injection contains the correct dosage of the active ingredient. Our production team uses advanced mixing techniques to ensure uniformity in the solution, which is critical to maintaining the efficacy and safety of the final product. This process is also designed to ensure that the formulation of Ceftiofur Sodium Injection 1000mg is stable and effective when given to patients.
Sterile Filtration and Filling into Vials
Once the formulation is complete, the next step is sterile filtration, which is done to remove any particulate matter, microorganisms, or impurities from the solution. This filtration ensures that the final product is free from contamination, which is a key requirement for injectable medications. After filtration, the solution is transferred into vials using automated filling systems. These systems are designed to accurately measure and fill each vial with the required amount of solution, ensuring consistency in every unit produced. After filling, the vials are sealed to maintain their sterility.
Lyophilization (Freeze Drying) for Lyophilized Injection
For specific types of Ceftiofur Sodium Injection, particularly those that require lyophilization (freeze-drying), the process involves removing the water content from the solution. This method is commonly used to extend the shelf life of the injection and make it more stable. The freeze-drying process involves freezing the product and then removing the moisture in a vacuum, leaving a thick dry powder that can be reconstituted before use. Lyophilized Ceftiofur Sodium Injection 1000mg offers extended storage stability and is preferred when a longer shelf life is required.
Stoppering and Sealing
After lyophilization, or once the sterile filtration and filling process is complete, the vials are sealed by stoppering them. Rubber stoppers are placed on each vial, and then the vials are sealed with aluminum caps to prevent contamination of the solution. This step is critical to maintaining the sterility and integrity of the injection, preventing exposure to external contaminants during storage and transportation.
Quality Control (QC) and Quality Assurance (QA) Testing
Before any product is released to the market, it undergoes a final round of Quality Control (QC) and Quality Assurance (QA) testing. This includes testing for the physical and chemical characteristics of Ceftiofur Sodium Injection 1000mg, ensuring that it meets all the necessary specifications for safety and potency. Our QA team ensures that the product is fully compliant with national and international pharmaceutical standards. These tests include checking for the correct concentration of active ingredients, sterility, and the absence of contaminants, ensuring that each batch of Ceftiofur Sodium Injection meets the highest quality standards.
Labeling and Packaging
Once the product passes all QC and QA tests, it moves on to the labeling and packaging stage. Each vial of Ceftiofur Sodium Injection 1000mg is carefully labeled with all necessary product information, including dosing instructions, storage conditions, expiration date, and safety warnings. The packaging is designed to protect the vials during transportation and storage, ensuring that the product arrives in perfect condition and is ready for use when needed. We take great care to ensure that labeling is clear, accurate, and in compliance with regulatory guidelines.
Storage and Storage
After labeling and packaging, the final product is stored in our secure warehousing and storage facilities. Storage conditions are carefully controlled to maintain product integrity, including temperature and humidity levels. We ensure that Ceftiofur Sodium Injection is stored in optimal conditions, thereby maintaining its stability and increasing its shelf life. Our warehouse is designed to accommodate large quantities of inventory, allowing us to maintain an efficient supply chain and respond quickly to demand.
Distribution and Supply Chain
Once the product is ready for distribution, we leverage a robust distribution and supply chain network to ensure that Ceftiofur Sodium Injection 1000mg reaches healthcare providers, veterinary clinics, and distributors across India. Our logistics team works closely with Ceftiofur Sodium Injection distributors in India and Ceftiofur Sodium Injection suppliers in India to ensure that the product is delivered efficiently and on time. We also ensure that Ceftiofur Sodium Injection in Delhi NCR and other regions has easy access to our products, thereby aiding healthcare systems across India. We constantly optimize our distribution network to meet the growing demand for our products while maintaining a commitment to quality and reliability.
Uses and Side Effects
Ceftiofur Sodium Injection 1000mg is primarily used for the treatment of bacterial infections in both humans and animals. It is effective against a wide range of gram-positive and gram-negative bacteria and is commonly used to treat infections such as pneumonia, urinary tract infections, and intra-abdominal infections. In veterinary medicine, it is commonly used to treat infections in livestock and pets. Like all antibiotics, Ceftiofur Sodium Injection 1000mg may cause side effects including allergic reactions, gastrointestinal problems, and local injection site reactions. It is important for healthcare providers and veterinarians to carefully monitor patients for any signs of adverse reactions and adjust treatment protocols as needed.
At R K Lifecare INC, We are committed to providing high-quality pharmaceutical products that support the health and well-being of patients. With Ceftiofur Sodium Injection 1000mg, We uphold our promise of excellence in the healthcare industry, ensuring that our products contribute to effective treatment regimes for bacterial infections in India and beyond.

12 Feb 2026

09 Feb 2026

07 Feb 2026
.jpg)
06 Feb 2026

04 Feb 2026