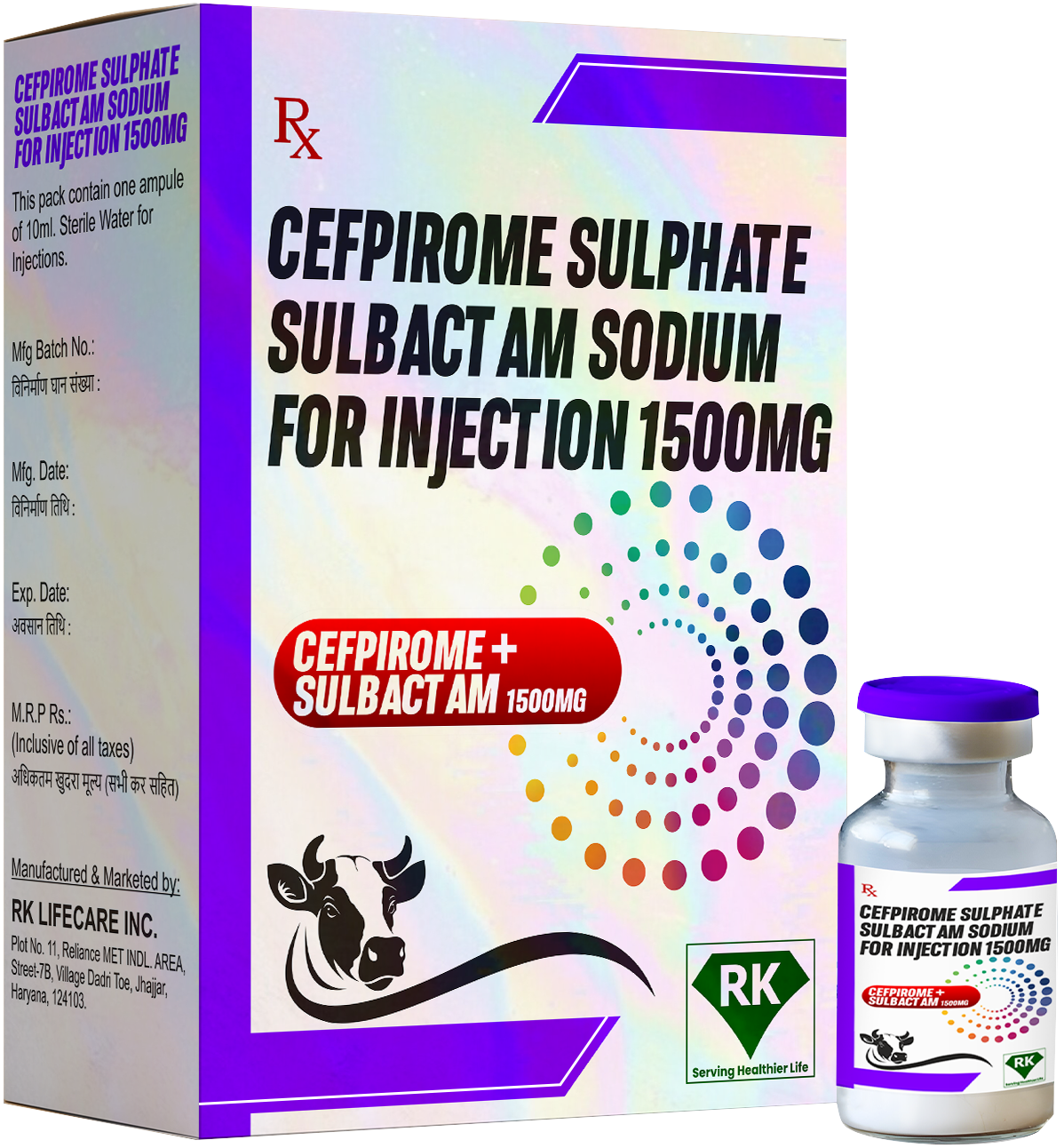
At R K Lifecare INC, We are at the forefront of the pharmaceutical industry as dedicated manufacturers and wholesalers of Cefpirome Sulphate Injection 1500mg and Sulbactam 1500mg for Injection. Based in India, we have earned a reputation for producing and supplying high-quality injectable antibiotics that play a vital role in the healthcare ecosystem.
Both Cefpirome Sulphate and Sulbactam are widely recognized for their effectiveness in treating a number of serious bacterial infections including respiratory tract infections, urinary tract infections, intra-abdominal infections, and skin and soft tissue infections. These injectable formulations are essential in clinical settings where patients require potent antibiotics to fight multi-drug resistant pathogens.
Cefpirome Sulphate is a fourth-generation cephalosporin, and Sulbactam is a beta-lactamase inhibitor that enhances the activity of certain antibiotics. Together, these two compounds provide an effective combination for treating severe infections, making them indispensable in critical care units, hospitals, and emergency medical care. As Cefpirome Sulphate Injection manufacturers in India, we take great pride in our manufacturing capabilities and processes, which are designed to ensure the highest quality and safety standards.
Our commitment to excellence is reflected in the meticulous steps we take to manufacture each batch of Cefpirome Sulphate 1500mg Injection and Sulbactam 1500mg for Injection. Each stage of production is closely monitored by our experienced team to ensure that the final product meets regulatory requirements and delivers the intended therapeutic results.
Our adherence to Good Manufacturing Practices (GMP) ensures that all our products are consistently manufactured according to the highest quality standards. As Cefpirome Sulphate Injection wholesalers in India, we recognize the importance of reliable distribution, ensuring that our products reach hospitals, pharmacies, and healthcare providers in a timely and efficient manner.
We are also proud to be a reliable Cefpirome Sulphate Injection supplier in India, serving customers across the country, including major healthcare hubs like Delhi NCR. Our extensive network of Cefpirome Sulphate Injection distributors in India ensures that our products are available across the country, meeting the needs of healthcare professionals and patients.
As a dedicated Cefpirome Sulphate Injection wholesaler in India, We also work closely with Sulbactam wholesalers in India, ensuring that healthcare providers have access to both components for effective treatment combinations. Our partnerships with Sulbactam suppliers in India further expand our reach, allowing us to deliver these lifesaving drugs where they are needed most.
At R K Lifecare INC, We believe that quality and reliability go hand in hand. Through advanced manufacturing techniques, a robust distribution system, and a deep commitment to patient care, we have established ourselves as a leader in the Indian pharmaceutical industry. With an unwavering focus on patient safety and satisfaction, we continue to contribute to the healthcare sector by supplying Cefpirome Sulphate Injection 1500mg and Sulbactam 1500mg for Injection to hospitals, clinics, and healthcare providers in India and beyond.
Labeling and Packaging
Proper labeling and packaging play a vital role in the overall safety and effectiveness of any pharmaceutical product. At R K Lifecare INC, We ensure that each vial of Cefpirome Sulphate Injection 1500mg and Sulbactam 1500mg for Injection is accurately labeled with all necessary details, including the drug's composition, instructions for use, dosage recommendations, storage guidelines, and any potential side effects.
We understand the importance of clear and concise labeling to reduce medication errors, so our labels are designed to be easily readable by healthcare professionals, with all necessary information highlighted in a straightforward format.
Our packaging is also designed with safety in mind. We use high-quality, tamper-proof packaging materials that provide an additional layer of protection for the product, ensuring it does not become contaminated during transit and storage. The packaging is designed to withstand the rigors of transportation, protecting the product from damage or exposure to harmful elements. Additionally, we ensure that the packaging is user-friendly, making it easier for healthcare providers to administer the medication.
Storage and Storage
Storage and storage of injectable products such as Cefpirome Sulphate Injection 1500mg and Sulbactam 1500mg for Injection requires careful attention to maintain their stability and efficacy. At RK Lifecare INC, We have invested in state-of-the-art storage facilities that are equipped with temperature and humidity control systems to ensure the preservation of our products’ potency.
Our storage areas are regularly monitored, with automated systems in place to track environmental conditions in real-time. These systems ensure that products are kept in optimal conditions until they are ready to be distributed to hospitals, clinics, and pharmacies.
We also implement strict inventory management protocols to ensure that products are properly rotated, and older stock is used first, reducing the chances of expiration or deterioration. By following these best practices, we can guarantee that every batch of Cefpirome Sulphate Injection 1500mg and Sulbactam 1500mg for Injection is stored safely, maintaining its high quality throughout its shelf life.
Distribution and Supply Chain At RK Lifecare INC, We have developed a highly efficient distribution and supply chain network that ensures timely delivery of Cefpirome Sulphate Injection 1500mg and Sulbactam 1500mg for Injection to healthcare facilities across India. We understand the urgency often involved in making life-saving medicines available, which is why we have streamlined our logistics to provide fast and reliable delivery.
From our manufacturing facilities to warehouses and distribution centers, our supply chain is designed for efficiency, flexibility, and reliability. Our extensive network of Cefpirome Sulphate Injection distributors in India and Sulbactam suppliers in India allows us to reach even the most remote areas, ensuring that our products are accessible to healthcare providers across the country. Be it Delhi NCR, metropolitan cities or rural areas, we are committed to making our products available wherever they are needed the most.
Our partnerships with Sulbactam wholesalers in India further enhance our distribution capabilities, ensuring that healthcare providers can access both components of the combination therapy in a seamless and efficient manner. Lyophilization (Freeze Drying) for Lyophilized Injection
For injectable drugs like Cefpirome Sulphate Injection 1500mg and Sulbactam 1500mg for Injection, the process of lyophilization (freeze drying) can be employed to enhance the stability of the product. Lyophilization involves removing the water content from the solution, resulting in a dry powder form that can be reconstituted with a diluent before use. This method is particularly useful for injectable products that need to be stored for long periods of time without compromising their efficacy.
The lyophilization process helps maintain the stability of active ingredients, prevent degradation and ensure a longer shelf life. Our facility is equipped with advanced lyophilization technology that ensures the highest quality standards for all our lyophilized products. Every lyophilized batch is thoroughly quality checked to confirm that it maintains the same level of efficacy as non-lyophilized formulations.
Stoppering and Sealing
Stoppering and sealing are critical processes in the production of injectable drugs, as they ensure the sterility and integrity of the product. After the vials are filled with Cefpirome Sulphate Injection 1500mg and Sulbactam 1500mg for Injection, the vials are sealed with rubber stoppers, which are then secured with aluminium seals. This process prevents any external contaminants from entering the vial and prevents potential damage to the product.
Our automated sealing equipment ensures that each vial is sealed properly, minimising the risk of human error and ensuring that every product that leaves our facility is ready for safe administration.
Procurement of Raw Materials
The quality of any pharmaceutical product depends largely on the quality of the raw materials used in its production. At RK Lifecare INC, We prioritise the procurement of high quality raw materials for both Cefpirome Sulphate and Sulbactam. We source our ingredients from reputable suppliers who meet stringent quality standards and provide complete documentation of the sourcing and testing of their ingredients.
Each batch of raw materials undergoes rigorous testing in our in-house laboratory to verify its quality and suitability for use in the manufacturing process. Only raw materials that pass these tests are used in the production of Cefpirome Sulfate Injection and Sulbactam for Injection, to ensure that the final product meets our high standards of safety and efficacy.
Quality Control (QC) of Raw Materials
To ensure the safety and efficacy of Cefpirome Sulfate Injection 1500mg and Sulbactam for Injection 1500mg, we perform thorough quality control (QC) checks on all raw materials before they are used in the manufacturing process. Our QC team tests each batch of raw materials for purity, potency and other required parameters. By maintaining rigorous QC standards, we ensure that only the highest quality ingredients are used in our products.
Manufacturing and Blending
The manufacturing and blending of cefpirome sulfate and sulbactam is carefully performed to ensure a homogeneous and stable final product. During this step, the active ingredients are mixed with excipients to form a uniform solution or suspension that is ready for sterile filtration and filling. Our manufacturing team works with precision to ensure that the composition of each batch is consistent, ensuring the effectiveness and safety of the final product.
Sterile Filtration and Filling into Vials
Once manufacturing is complete, the solution undergoes sterile filtration to remove any impurities or particulate matter. The filtered solution is then filled into sterile vials under aseptic conditions to maintain its sterility. This step is critical in ensuring that the product is free from contamination and ready for safe use in clinical settings.
Quality Control (QC) and Quality Assurance (QA) Testing
After the product is manufactured, we conduct a final series of Quality Control (QC) and Quality Assurance (QA) tests to ensure that it meets all regulatory standards and specifications. These tests include checking for sterility, potency, and stability. Only products that pass these rigorous tests are released for distribution.
Uses and Side Effects
Cefpirome Sulphate Injection 1500mg and Sulbactam Injection 1500mg are commonly used to treat a wide range of bacterial infections including pneumonia, urinary tract infections,

22 Jan 2026
20 Jan 2026

19 Jan 2026

13 Jan 2026

08 Jan 2026