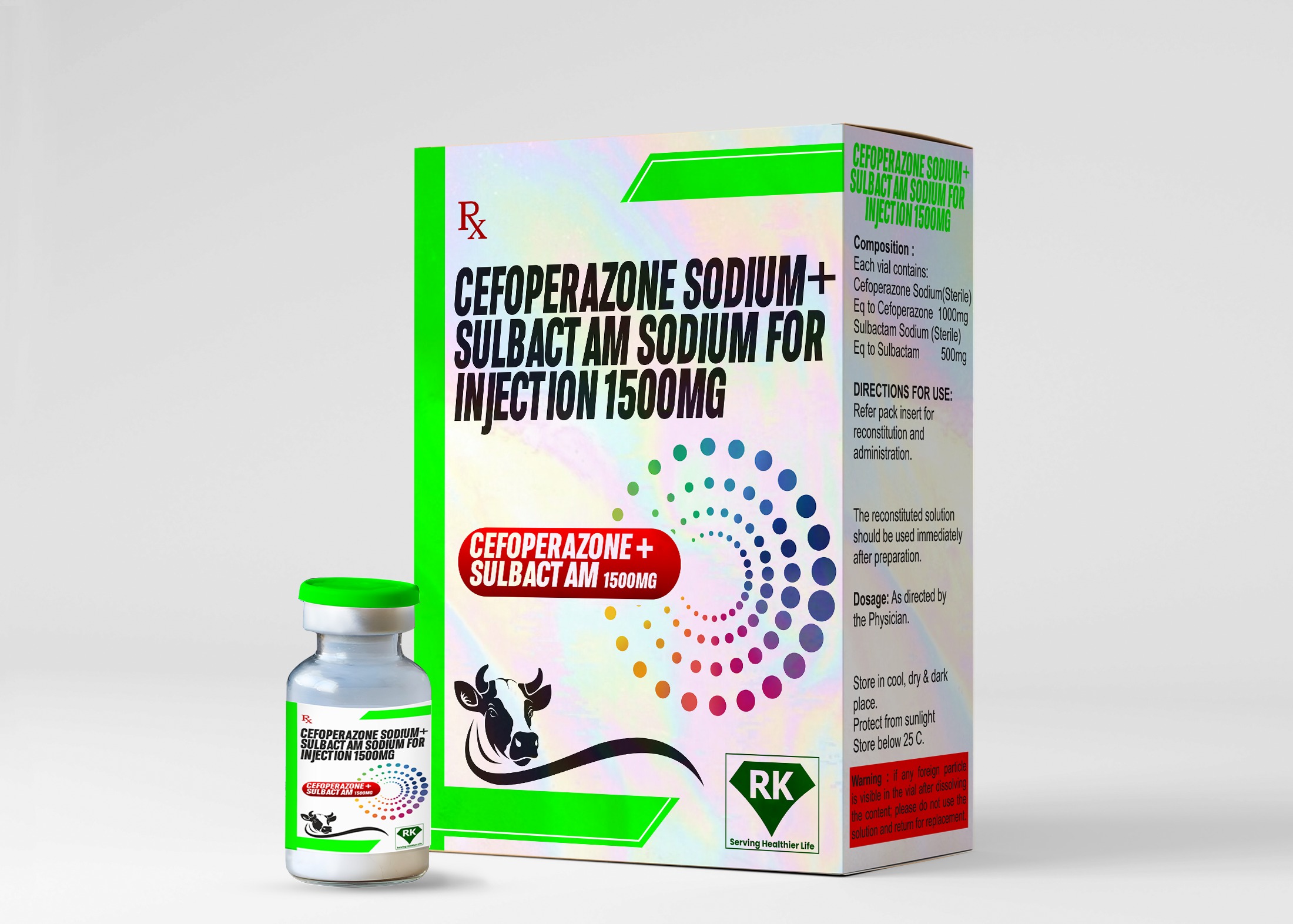
At R K Lifecare INC, We are proud to be the leading Cefoperazone Sodium and Sulbactam Sodium for Injection 1500mg in India. With a commitment to quality, innovation, and patient safety, we manufacture and distribute one of the most effective combinations of antibiotics in the market today.
A third-generation cephalosporin antibiotic, Cefoperazone Sodium is known for its broad-spectrum activity against both gram-positive and gram-negative bacteria, making it an essential tool in the treatment of serious bacterial infections.
By combining Cefoperazone Sodium with Sulbactam Sodium, a potent beta-lactamase inhibitor, we enhance the effectiveness of the antibiotic, especially against resistant strains of bacteria that may otherwise be difficult to treat with standard antibiotics.
This combination therapy is especially beneficial in the hospital setting, where the risk of multi-drug resistant infections is high. Our commitment to producing the highest quality Cefoperazone Sodium and Sulbactam Sodium 1500mg for Injection is evident in our adherence to strict manufacturing protocols.
As Cefoperazone Sodium Injection manufacturers in India, We use state-of-the-art facilities equipped with the latest technology to ensure that each batch is consistent, safe, and effective. Our manufacturing processes adhere to the highest standards, including Good Manufacturing Practice (GMP), to guarantee product quality. At R K Lifecare INC, We believe in the power of quality assurance and constantly strive to improve our processes to meet the ever-changing demands of the healthcare industry.
Our expertise goes beyond manufacturing, as we are also a trusted Cefoperazone Sodium Injection wholesaler in India. We supply our products to hospitals, clinics, and pharmaceutical distributors across the country. As a leading Cefoperazone Sodium Injection distributor in India, we have built a reliable network of partners to ensure that Cefoperazone Sodium Injection is consistently available in Delhi NCR and other regions.
This distribution network allows healthcare providers to access essential medicines when they need them most, ensuring that patients receive timely treatment. As a Cefoperazone Sodium Injection supplier in India, we understand the importance of maintaining the right supply chain to ensure the constant availability of this lifesaving drug.
In addition to our role as a manufacturer of Cefoperazone Sodium Injection, we are also proud to be a leading Sulbactam Sodium manufacturer in India. Sulbactam sodium is a key ingredient in our combination product, as it enhances the antibacterial activity of Cefoperazone Sodium by inhibiting bacterial enzymes that can render antibiotics ineffective. By producing Sulbactam Sodium in India, we ensure that we can maintain the highest quality standards and meet the growing demand for this important drug.
As a wholesaler of Sulbactam Sodium in India, we work closely with distributors to ensure that Sulbactam Sodium in Delhi NCR and across the country is always available to healthcare providers and hospitals that rely on it to treat infections.
The quality of our products is paramount, and we understand that the production process plays a vital role in ensuring that our medicines are both safe and effective. Below, we take a detailed look at the processes and procedures involved in the production of Cefoperazone Sodium and Sulbactam Sodium 1500mg for Injection.
Procurement of Raw Materials
The production of Cefoperazone Sodium and Sulbactam Sodium 1500mg for Injection begins with the careful procurement of raw materials. At R K Lifecare INC, We ensure that all ingredients, including Cefoperazone Sodium and Sulbactam Sodium, are sourced from reliable and trustworthy suppliers.
These suppliers are selected based on their ability to provide high-quality, pure and effective ingredients that meet international standards. The procurement process involves rigorous checking for authenticity, potency and purity to ensure that only the best ingredients are used in the production of our injectables.
Quality Control (QC) of Raw Materials
Once the raw materials are procured, they undergo stringent quality control (QC) testing. This step is crucial to ensure that the ingredients meet the required specifications and are free from impurities. At R K Lifecare INC, We have a dedicated team of quality control experts who perform thorough testing on each batch of raw materials. This includes testing for identity, strength, purity, and overall quality. The goal is to ensure that each ingredient used in the production of Cefoperazone Sodium and Sulbactam Sodium for Injection 1500mg meets the highest standards and that the final product is safe and effective for use.
Formulation and Blending
After the raw materials have undergone QC testing, the next step in the process is formulation and blending. This is where the active ingredients, Cefoperazone Sodium and Sulbactam Sodium, are blended together in precise proportions. During this step, we ensure that the ingredients are evenly distributed throughout the mixture, creating a homogeneous solution. The mixing process is carefully controlled to ensure consistency and uniformity in each batch. Our manufacturing team uses the latest technology to perform this process with precision and accuracy, ensuring that the final product is of the highest quality.
Sterile Filtration and Filling into Vials
Once the manufacturing is complete, the next critical step is sterile filtration. This process removes any potential contaminants from the solution, ensuring that the injection remains sterile and safe for use. Once the solution is sterilized, it is filled into vials under aseptic conditions. The filling process is automated and done very carefully to ensure that each vial contains the correct dosage of Cefoperazone Sodium and Sulbactam Sodium 1500mg for Injection. Vials are filled in a clean room environment, and every step is monitored to maintain sterility and prevent contamination.
Quality Control (QC) and Quality Assurance (QA) Testing
Once the vials are filled, the product undergoes further quality control (QC) and quality assurance (QA) tests. These tests include checking sterility, potency, and stability, ensuring the product will remain effective throughout its shelf life. We also perform a number of other tests, such as ensuring the product is free of particles and that it meets the required specifications for pH and appearance. These tests are performed by our highly trained QC and QA teams, who are committed to ensuring that every vial of Cefoperazone Sodium and Sulbactam Sodium for Injection 1500mg is safe for patient use.
Labeling and Packaging
After passing the required quality tests, the final product is labeled and packaged. Labeling and packaging are essential steps in the manufacturing process because they ensure the product is properly identified and healthcare providers have all the necessary information for safe and effective use. Labeling includes important information such as dosage, administration instructions, expiration date, and potential side effects. Packaging is designed to protect vials from contamination and damage during transportation and storage. At R K Lifecare INC, We ensure all packaging materials comply with regulatory standards and labeling is clear, accurate, and easy to understand.
Storage and Warehouse
After the product is packaged, it is stored in our state-of-the-art storage and warehouse facilities. These facilities are designed to maintain the required temperature and humidity conditions to ensure product stability and efficacy. We use temperature-controlled storage areas to ensure that Cefoperazone Sodium and Sulbactam Sodium Injection 1500mg remains in optimal storage conditions throughout its shelf life. Our warehouse is equipped with the latest inventory management systems to ensure that products are tracked and accounted for at all times.
Distribution and Supply Chain
R K Lifecare INC has established a strong distribution and supply chain network to ensure that our products reach healthcare providers in a timely and efficient manner. As a leading Cefoperazone Sodium Injection wholesaler in India and Sulbactam Sodium distributor in India, we have partnered with a wide network of distributors and dealers to ensure that our products are readily available. We maintain strong relationships with Cefoperazone Sodium Injection distributors in India and Sulbactam Sodium suppliers in India, enabling us to meet the needs of hospitals, pharmacies and clinics across the country, including Delhi NCR.
Lyophilization (Freeze Drying) (For Lyophilized Injections Only)
For specific formulations, R K Lifecare INC uses lyophilization (freeze drying) to maintain product stability. This process removes moisture from the solution, keeping it stable and effective for longer periods of time. Lyophilization is particularly useful for injectable formulations, as it increases shelf life without compromising the efficacy of the active ingredients. While Cefoperazone Sodium and Sulbactam Sodium for 1500mg Injection do not always undergo lyophilization, it remains a key technique for other injectable products in our portfolio.
Stoppering and Sealing
The final step in the production process is stoppering and sealing. This ensures that each vial of Cefoperazone Sodium and Sulbactam Sodium Injection 1500mg is properly sealed and protected from contamination. The stopper is securely fitted to each vial, and the seal is checked to ensure that it is tamper-proof, providing additional assurance of product integrity.
Uses and Side Effects
Cefoperazone Sodium and Sulbactam Sodium Injection 1500mg is primarily used to treat severe bacterial infections, including respiratory tract infections, urinary tract infections, skin and soft tissue infections, and intra-abdominal infections. The combination of Cefoperazone Sodium, a broad-spectrum cephalosporin, and Sulbactam Sodium, a beta-lactamase inhibitor, provides enhanced antibacterial activity against resistant bacterial strains.
Like all medicines, **Cefoperazone Sodium and Sulbactam Sodium Injection 1500mg

22 Jan 2026
20 Jan 2026

19 Jan 2026

13 Jan 2026

08 Jan 2026