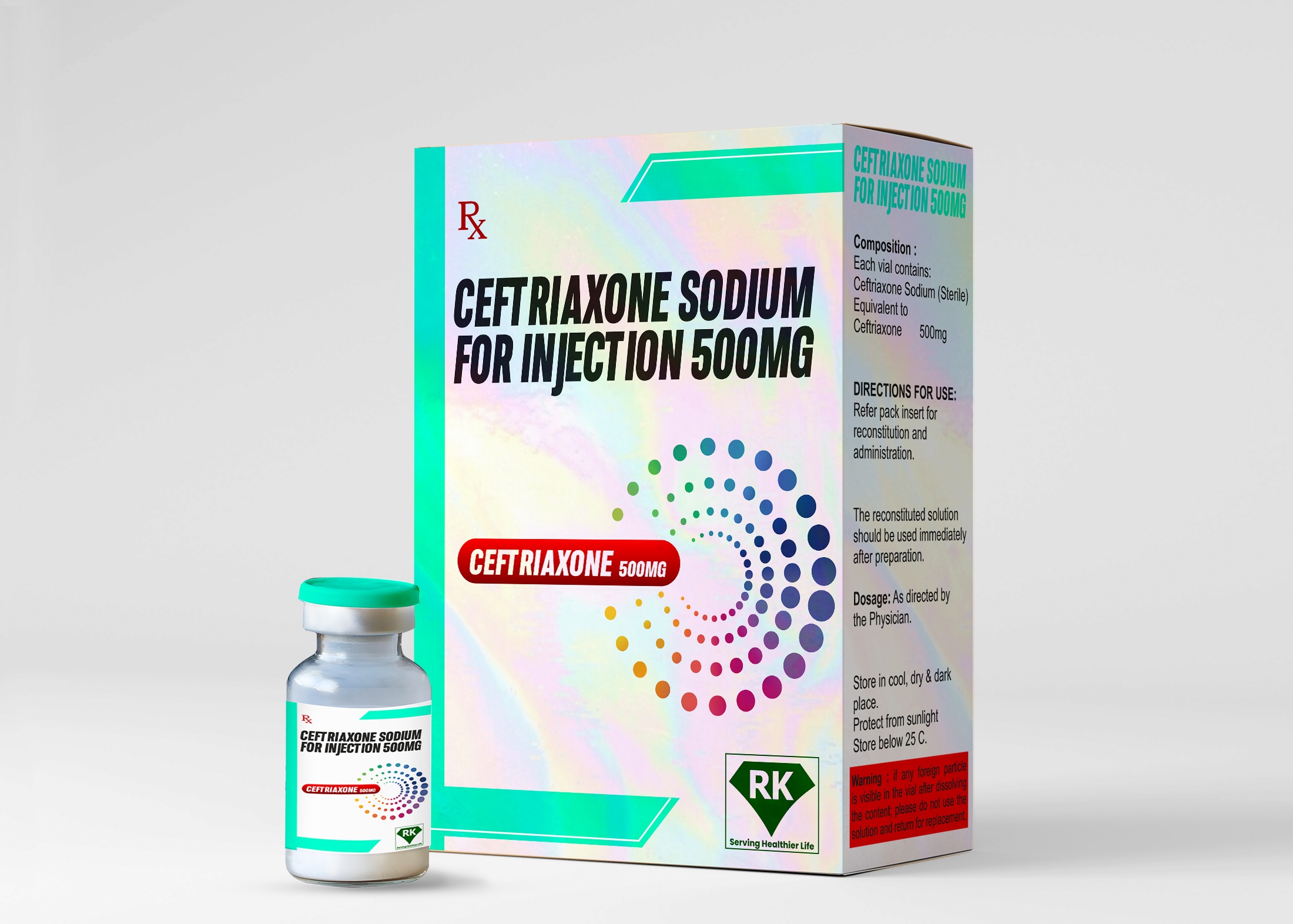
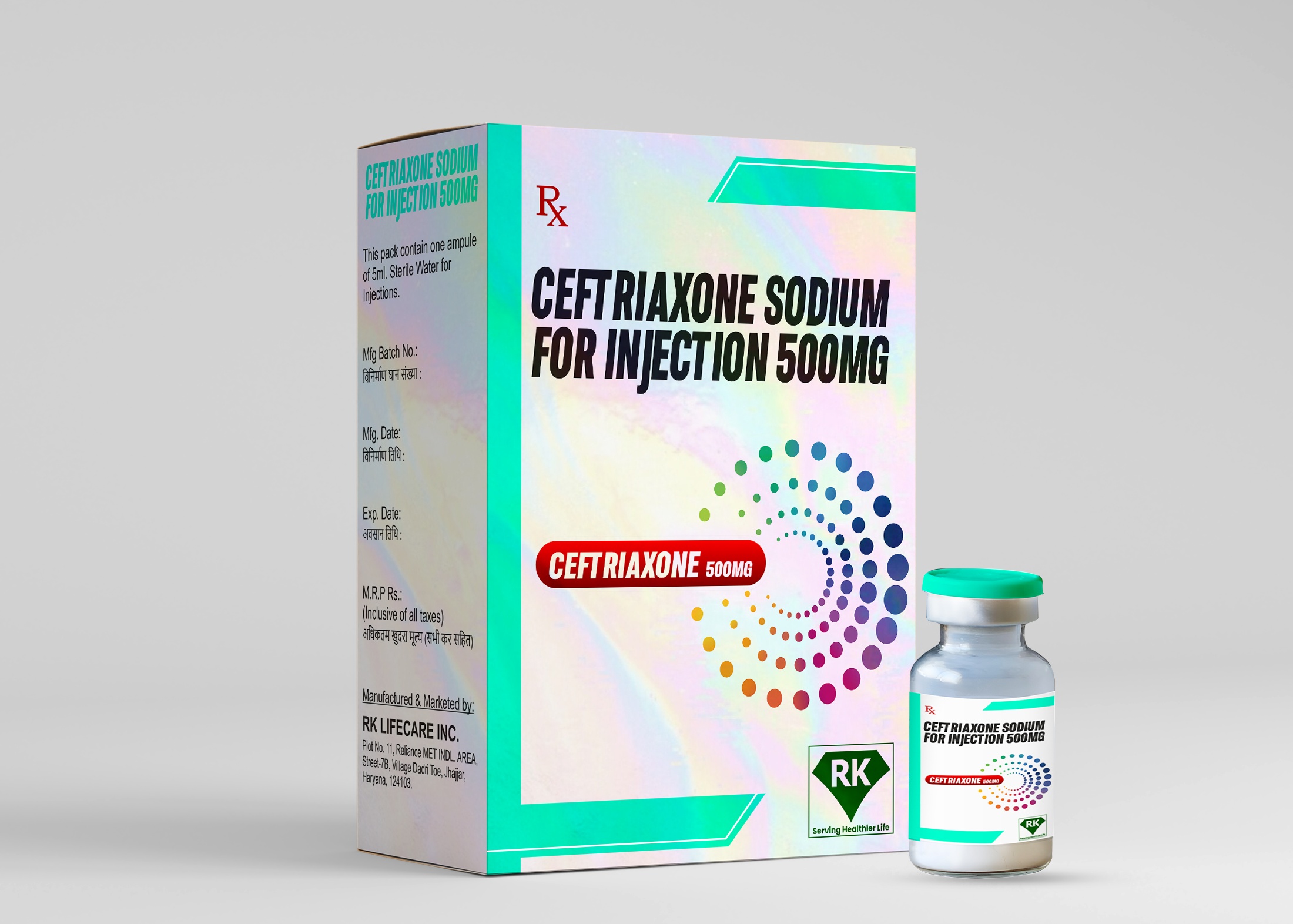
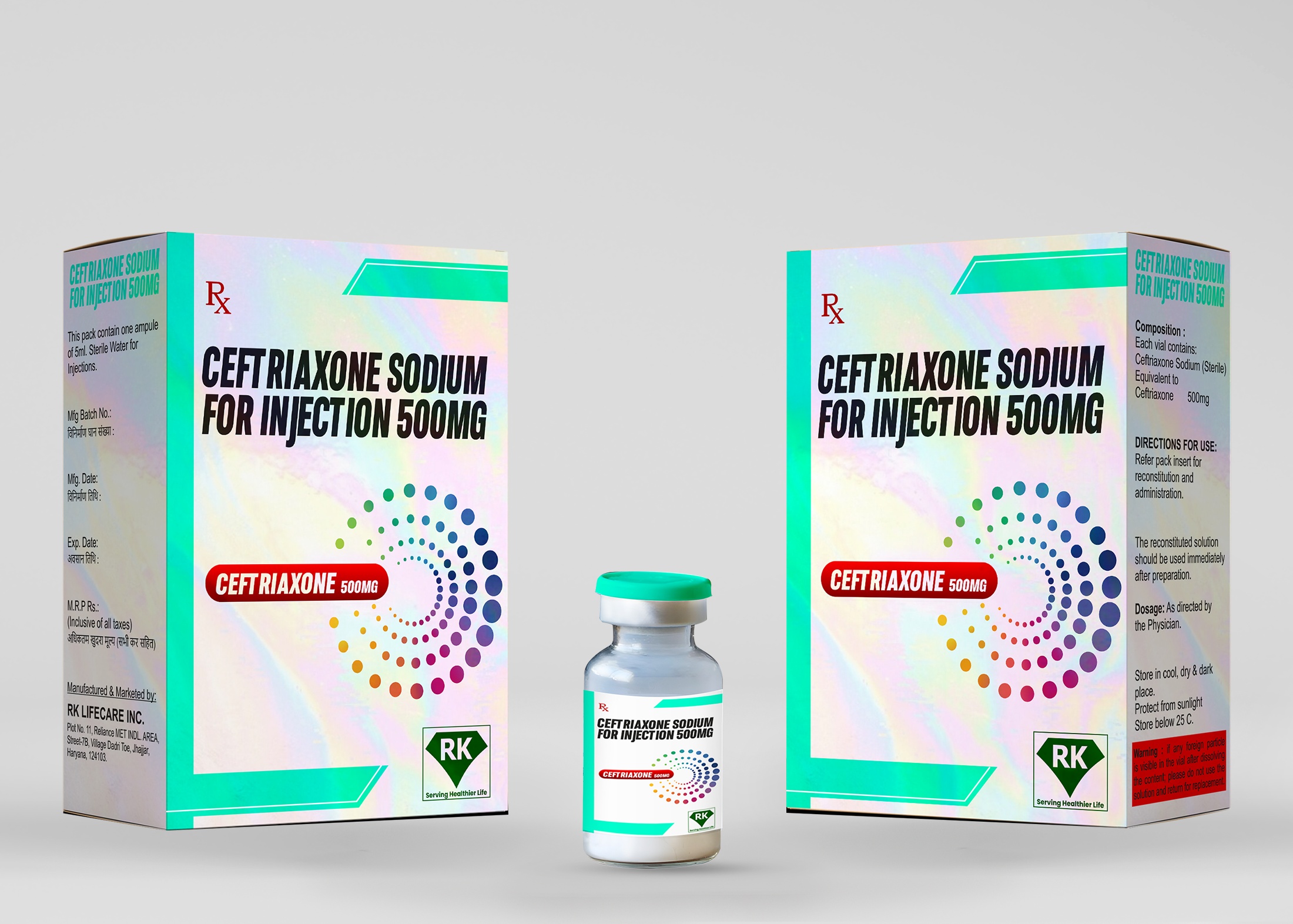
R K Lifecare INC is a leading ceftriaxone injection manufacturer in India, specializing in the production of high-quality Ceftriaxone Sodium for Injection 500mg. Our state-of-the-art manufacturing facility adheres to stringent WHO-GMP and regulatory guidelines, ensuring that our products meet the highest standards of safety, efficacy, and quality.
This document provides an in-depth look at our Ceftriaxone Sodium Injection manufacturing process, including formulation, production steps, quality control, packaging, marketing, and distribution.
1. Formulation of Ceftriaxone Sodium for Injection 500mg
Active Pharmaceutical Ingredient (API)
The key component of Ceftriaxone Sodium for Injection 500mg is Ceftriaxone Sodium, a broad-spectrum cephalosporin antibiotic used to treat a variety of bacterial infections.
Composition
-
Ceftriaxone Sodium equivalent to Ceftriaxone 500mg
-
Sterile excipients
-
Stabilizers
Mechanism of Action
Ceftriaxone works by inhibiting bacterial cell wall synthesis, leading to bacterial destruction. It is effective against Gram-positive and Gram-negative organisms and is widely used for treating infections such as pneumonia, meningitis, and urinary tract infections.
2. Manufacturing Process of Ceftriaxone Sodium for Injection 500mg
Step 1: Raw Material Procurement and Testing
We source high-quality Ceftriaxone Sodium and excipients from certified vendors. Before use, all materials undergo rigorous testing to ensure purity, sterility, and compliance with pharmacopoeial standards.
Step 2: Sterile Powder Preparation
-
Precise weighing and mixing of raw materials in a controlled environment.
-
The mixture is processed under sterile conditions to prevent contamination.
-
The formulation undergoes vacuum drying to eliminate excess moisture.
Step 3: Filling and Sealing
-
The sterile Ceftriaxone Sodium for Injection 500mg powder is accurately measured and filled into vials.
-
Each vial is sealed with rubber stoppers and aluminum caps to maintain sterility and stability.
Step 4: Quality Control and Testing
Every batch undergoes comprehensive quality control tests, including:
-
Sterility Testing
-
Potency Testing
-
pH and Chemical Stability Analysis
-
Particulate Matter Examination
Step 5: Packaging and Labeling
-
Vials are packed in cartons with patient information leaflets.
-
Proper labeling includes batch number, expiry date, dosage, and storage conditions.
3. Distribution and Supply Chain of Ceftriaxone Sodium for Injection 500mg
R K Lifecare INC follows a robust distribution process to ensure the timely delivery of Ceftriaxone Sodium Injection across India and international markets.
Distribution Network
-
Ceftriaxone injection wholesalers in Delhi NCR distribute our products to hospitals, clinics, and pharmacies.
-
We partner with ceftriaxone injection wholesalers in India to enhance accessibility nationwide.
-
Our international logistics ensure seamless supply chain management for exports.
Cold Chain Logistics
-
Ceftriaxone Sodium Injection requires controlled temperature storage and transportation.
-
We collaborate with validated logistics partners to ensure the safe and effective delivery of our products.
Ceftriaxone Injection Wholesale Partnerships
We work with reputed ceftriaxone injection wholesalers to ensure that Ceftriaxone Sodium for Injection 500mg is readily available in healthcare institutions at competitive prices.
4. Market Chain and Demand for Ceftriaxone Sodium for Injection 500mg
Market Analysis
The demand for Ceftriaxone Sodium Injection is consistently high due to its effectiveness against severe bacterial infections. Ceftriaxone injection manufacturers in India play a crucial role in supplying both domestic and international healthcare sectors.
Key Buyers
-
Hospitals and Healthcare Institutions
-
Government Health Programs
-
Retail Pharmacies
-
Veterinary Clinics
Competitive Advantages of R K Lifecare INC
-
High-Quality Manufacturing Standards: As a leading ceftriaxone injection manufacturer in India, we ensure strict quality control and compliance.
-
Cost-Effective Solutions: Our efficient production processes allow us to offer ceftriaxone injection wholesale at competitive prices.
-
Regulatory Compliance: We meet all necessary regulatory approvals and quality certifications.
-
Widespread Distribution Network: Our partnership with ceftriaxone injection wholesalers in India ensures wide market reach.
5. Regulatory Compliance and Certifications
Good Manufacturing Practices (GMP) Compliance
-
We strictly adhere to WHO-GMP guidelines for producing Ceftriaxone Sodium for Injection 500mg.
-
Regular audits and inspections ensure compliance with international regulatory standards.
Drug Regulatory Approvals
-
Our products are approved by DCGI (Drug Controller General of India) and other global regulatory bodies.
-
We maintain complete documentation for licensing and exports.
Quality Assurance Measures
-
In-process quality control checks at every stage of production.
-
Final batch release testing to ensure pharmacopoeial compliance.
6. Future Growth Strategies for Ceftriaxone Injection Manufacturing
Expansion Plans
-
Increasing production capacity to meet rising demand.
-
Strengthening partnerships with ceftriaxone injection wholesalers in Delhi NCR for better market penetration.
-
Exploring international markets to enhance global presence.
Innovations in Drug Delivery
-
Researching advanced formulations for enhanced efficacy.
-
Developing combination therapies with Ceftriaxone Sodium Injection to improve treatment outcomes.
Sustainability Initiatives
-
Implementing eco-friendly packaging solutions.
-
Reducing carbon footprint through energy-efficient manufacturing practices.
Conclusion
As a top ceftriaxone injection manufacturer in India, R K Lifecare INC is committed to delivering high-quality Ceftriaxone Sodium for Injection 500mg with superior efficacy and reliability. Through stringent quality control, an extensive distribution network, and continuous innovation, we remain at the forefront of pharmaceutical excellence.
For inquiries related to ceftriaxone injection wholesale, partnerships, or bulk orders, contact us today. We look forward to providing premium-quality Ceftriaxone Sodium Injection to healthcare professionals worldwide.
08 Dec 2025
03 Dec 2025

02 Dec 2025

31 Oct 2025

30 Oct 2025