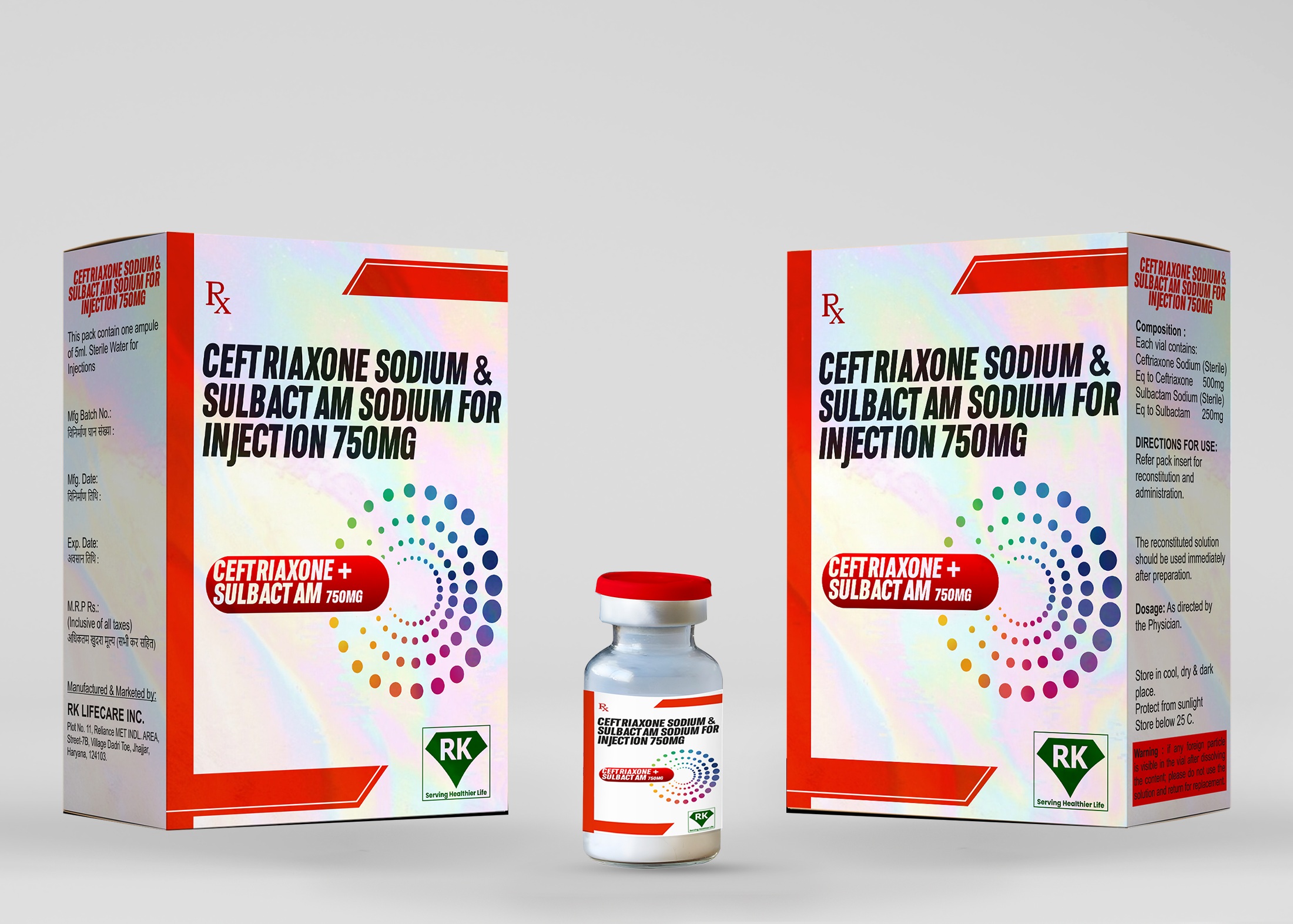
R K Lifecare INC is a leading Ceftriaxone Injection manufacturer in India, specializing in producing high quality Ceftriaxone Sodium and Sulbactam Sodium 750mg for injection. As a leading Ceftriaxone Injection manufacturer in India, we ensure that our products meet stringent quality standards and regulatory guidelines. Our commitment to excellence makes us one of the most trusted Ceftriaxone Injection manufacturers supplying to hospitals, pharmacies and wholesalers in India and beyond.
Composition and Formula
Ceftriaxone Sodium and Sulbactam Sodium Injection 750mg is a combination antibiotic designed to treat serious bacterial infections. This formulation contains:
-
Ceftriaxone Sodium 500mg – A third-generation cephalosporin antibiotic that inhibits bacterial cell wall synthesis.
-
Sulbactam Sodium 250mg – A beta-lactamase inhibitor that enhances the efficacy of Ceftriaxone Injection by preventing bacterial resistance.
Manufacturing Process
1. Procurement of Raw Materials
The manufacturing process begins with sourcing high-quality Ceftriaxone Sodium and Sulbactam Sodium from certified suppliers. These raw materials undergo stringent testing to ensure purity, stability, and effectiveness before production.
2. Pre-Formulation Studies
Prior to manufacturing, pre-formulation studies determine the compatibility and stability of Ceftriaxone Sodium Injection 750mg. This step ensures that the drug maintains its potency and therapeutic effectiveness.
3. Preparation of Sterile Environment
To prevent contamination, production is carried out in a sterile environment with advanced cleanroom technology. Our facility features the following:
-
HEPA-filtered air supply
-
Aseptic processing areas
-
Stringent environmental controls
4. Formulation and Mixing
The active pharmaceutical ingredients (APIs) - ceftriaxone sodium and sulbactam sodium - are weighed and mixed in precise proportions to ensure an accurate dose of Ceftriaxone Injection 750 mg.
5. Sterile Filtration
The solution is passed through a sterile filtration system to remove any contaminants or particulate matter, ensuring a high level of purity.
6. Filling and Sealing of Vials
The filtered solution is aseptically filled into vials using automatic filling machines. The vials are immediately sealed to maintain sterility and prevent deterioration.
7. Lyophilization (Freeze-Drying)
To increase the stability and shelf life of Ceftriaxone Sodium for Injection, vials undergo freeze-drying. This removes moisture while maintaining the efficacy of the drug.
8. Quality Control and Testing
Each batch undergoes rigorous quality control tests, including:
-
Assay for potency
-
Sterility and pyrogen testing
-
pH analysis
-
Moisture content evaluation These tests ensure that the final product meets pharmacopoeial and regulatory standards.
9. Packaging and Labeling
The final vials are imprinted with necessary product information such as batch number, manufacturing and expiry dates, and storage instructions. The vials are then securely packaged for distribution.
Distribution and Market Chain of Ceftriaxone Sodium & Sulbactam Sodium for Injection 750mg
1. Wholesalers and Distributors
As one of the top Ceftriaxone Injection manufacturers in India, We supply bulk quantities to authorized wholesalers and distributors. Our well-established distribution network ensures that Ceftriaxone Sodium Injection is available across India, including Ceftriaxone Injection wholesalers in Delhi NCR.
2. Supply to Hospitals and Pharmacies
We provide Ceftriaxone Sodium and Sulbactam Sodium 750mg for Injection to hospitals, nursing homes and retail pharmacies, ensuring timely availability for patient treatment. As a trusted Ceftriaxone Sodium Injection manufacturer in India, We prioritize efficiency and reliability across supply chains.
3. Export and International Market
Our Ceftriaxone Injection 750mg is also exported to global markets adhering to international quality and safety standards. As a reliable exporter, we ensure that our Ceftriaxone Injection bulk supply meets the needs of international buyers.
Why Choose R K Lifecare INC?
-
High-Quality Manufacturing: We follow strict GMP and WHO guidelines to ensure the best standards in Ceftriaxone Sodium for Injection production.
-
Competitive Pricing: As a leading ceftriaxone injection wholesale supplier, we offer cost-effective pricing while maintaining superior quality.
-
Robust Supply Chain: We ensure seamless availability through a strong distribution network, supporting ceftriaxone injection wholesalers in India and beyond.
-
Regulatory Compliance: Our products meet all regulatory approvals for safety, efficacy, and quality assurance.
Conclusion
R K Lifecare INC is one of the most trusted Ceftriaxone Injection manufacturers in India, specializing in Ceftriaxone Sodium and 750mg Sulbactam Sodium for Injection. Our advanced manufacturing process, strict quality control and efficient distribution network make us a reliable partner for hospitals, wholesalers and global pharmaceutical markets. Whether you are looking for Ceftriaxone Injection wholesalers in Delhi NCR or looking for bulk supplies across India, we provide high quality pharmaceutical solutions to meet your needs.

21 Feb 2026

19 Feb 2026

17 Feb 2026

16 Feb 2026

14 Feb 2026